-
PDF
- Split View
-
Views
-
Cite
Cite
Zainulabeuddin Syed, Walter S. Leal, Maxillary Palps Are Broad Spectrum Odorant Detectors in Culex quinquefasciatus, Chemical Senses, Volume 32, Issue 8, October 2007, Pages 727–738, https://doi.org/10.1093/chemse/bjm040
Close - Share Icon Share
Abstract
A single type of olfactory sensilla on maxillary palps in many species of mosquitoes houses a very sensitive olfactory receptor neuron (ORN) for carbon dioxide reception. We performed extensive single sensillum recordings from this peg sensillum in Culex quinquefasciatus and have characterized the response threshold and kinetics for CO2 reception, with a detection threshold less than the CO2 concentration in the atmosphere. This ORN responded in a tonic mode to lower concentrations of CO2, whereas higher concentrations generated a phasic-tonic mode of action potential firing. Sensillum potentials accurately represented the response magnitude and kinetics of carbon dioxide–elicited excitatory responses. Stimulation of these ORNs with human breath, a complex mixture of mosquito kairomones and up to 4.5% CO2, elicited excitatory responses that were reliably detected by CO2-sensitive ORNs. Another ORN housed in these sensilla responded to 1-octen-3-ol and to various plant-derived compounds, particularly floral and green leaf volatiles. This ORN showed remarkable sensitivity to the natural enantiomer, (R)-(−)-1-octen-3-ol, rivaling pheromone-detecting ORNs in moths. Maximum neuronal response was elicited with a 10 ng dose. A biological, ecological role of maxillary palps in detection of plant- and nectar-related sources is proposed.
Introduction
Mosquitoes are one of the most important groups of insects affecting humans and animal health. They occupy diverse habitats, feed on a variety of animals, and transmit life-threatening diseases. West Nile virus, which causes encephalitis in both humans and animals, was introduced in the North America in 1999 and has been spreading across the Unites States (Anonymous 2006). Host and habitat location are mainly mediated by olfactory cues (Bowen 1996; Takken and Knols 1999; Zwiebel and Takken 2004). Olfaction in mosquitoes is mediated by elaborate olfactory appendages, antennae, and maxillary palps that carry a variety of structures called sensilla. These sensilla house olfactory receptor neurons (ORNs) in which olfactory receptor proteins (ORs) are embedded. A plethora of chemicals originating from skin, breath, plant/nectar, and oviposition sites are detected by these ORNs (Clements 1999). There are approximately 2000 antennal neurons housed in a variety of sensilla in Aedes aegypti, over 90% of which have olfactory function (McIver 1982). Host detection in mosquitoes starts with interactions between odorants and distinct subpopulation of ORs present on the dendritic membrane of ORNs. In Anopheles gambiae, at least 79 olfactory receptors have been identified, along with a highly conserved receptor AgOr7 that is expressed in both antenna and palps (Pitts et al. 2004). Another group of proteins, odorant-binding proteins (OBPs), are present in large amounts in the sensillum lymph surrounding the ORNs and are hypothesized to participate in the early perireceptor events in olfaction (Leal 2005). Several OBPs have been isolated and cloned from mosquitoes (Biessmann et al. 2002; Ishida et al. 2002, 2003; Vogt 2002; Xu et al. 2003; Ishida et al. 2004).
Carbon dioxide has long been used as an attractant for various mosquito species (Mboera and Takken 1997). CO2 is present in the atmosphere at approximately 350 ppm, a concentration that can fluctuate considerably with time of day and between different habitats (Gillies 1980; Zollner et al. 2004). Human breath is composed of 4–5% CO2 released at the rate of 275 ml/min (Gillies 1980). A single type of olfactory sensillum on the last distal (fourth) segment of maxillary palps was identified and termed as bulb organs (since then variously termed as peg sensilla, capitate pegs, or simply maxillary palp basiconic sensilla (McIver and Charlton 1970).
Extracellular single-cell recordings from these peg sensilla on maxillary palps in A. aegypti demonstrated that an ORN in these sensilla responds to CO2 with very high sensitivity (Kellogg 1970). Further characterization of this sensillum by ultrastructural methods identified 3 neurons, one of them profusely branched and have electron-lucid, light cytoplasm (McIver 1972). Later studies have shown that a number of insect species possess CO2-sensitive ORNs that resemble this profusely branched pattern (Stange and Stowe 1999). A clear proof for the role of the maxillary palp came after the demonstration that ablation of palps in Culex quinquefasciatus renders females unable to trace a CO2 source in a wind tunnel, whereas the ability to reach a hand as an attractive source was unaltered in these palpectomized females (Omer and Gillies 1971). Tracing of axonal projections from maxillary palps in A. aegypti revealed a very distinct glomerulus within the ipsilateral antennal lobe (Anton 1996; Distler and Boeckh 1997), though it is hard to conclude from these studies if the staining was taken up only by the CO2-sensitive ORN. Recent ultrastructural investigation in A. aegypti, however, has revealed afferent fibers from the maxillary palps nerve innervating 3 separate mediodorsal glomeruli (MD1–3) in both sexes (Ignell et al. 2005).
A detailed study on the physiology of CO2-sensitive ORNs in A. aegypti has revealed that an ORN with the biggest amplitude in the palpal basiconic sensilla responds to augmentation of CO2 by increasing the spontaneous firing rate and that these ORNs have a response threshold of approximately 300 ppm (Grant et al. 1995). This study further showed that the response to a pulse of a given concentration of CO2 is independent of the background level of CO2, up until that level is equal to or greater than the concentration of the stimulus pulse, thus acting as absolute CO2 detectors. Several studies have characterized the physiological properties of CO2-sensitive ORNs in other hematophagous arthropods like tsetse flies (Bogner 1992), ticks (Steullet and Guerin 1992), and midges (Grant and Kline 2003). There was a suggestion that one of the 2 ORNs colocalized with CO2-sensitive ORN in the A. aegypti palps detects other compounds (Grant and O'Connell 1996). A detailed study on the role of all the ORNs present in the peg sensilla is now warranted given the growing molecular evidence, suggesting that maxillary palps house olfactory receptors in addition to the highly conserved receptor AgOr7 in A. gambiae (Pitts et al. 2004), A. aegypti (Melo et al. 2004), and C. quinquefasciatus (Xia and Zwiebel 2006). Additionally, CO2-sensitive ORNs do not express this conserved receptor (Larsson et al. 2004; Jones et al. 2007). We asked the questions if other ORNs present in these sensilla detect any of the host or nectar-related compounds. We also characterized the response thresholds and kinetics of CO2-sensitive ORN of C. quinquefasciatus Say (Diptera: Culicidae). This report is part of the elaborate electrophysiological study of the mosquito ORNs from all the antennal and palpal sensilla as an initial step in understanding how the physiological properties of ORNs might participate in encoding odors. Here we report the results from maxillary palp sensilla that house 3 ORNs, 2 of which responding to a wide array of chemicals.
Materials and methods
Scanning electron microscopy
Female mosquito heads were fixated in acetone for 4 days and then subjected to critical point drying on Denton vacuum desk II. Heads were coated with gold for 120 s (deposits ca. 40 nm) in a Tousimis samdri-780A gold sputter and later observed under a Hitachi S3500N scanning electron microscopy.
Insects
Laboratory reared C. quinquefasciatus were used throughout this study. The colony was originally started from adult mosquitoes collected in Merced, CA, in the 1950s and has been maintained under laboratory conditions since 1984 at Kearney laboratory, University of California, Davis. Adults were maintained in cages and had unlimited access to a 10% sucrose solution. Females were allowed to engorge on restrained mice for 30 min once a week to facilitate oviposition. A water cup was placed in the cage for oviposition, and egg rafts were allowed to hatch in a tray. Early larval instars were provided with 10% liver powder solution and ground rodent diet, and late instars were fed on ground rodent diet ad lib until pupation. For electrophysiology, host-seeking adults from the colony that were either blood fed and had laid eggs or only sugar fed were chosen. No differences in responses were noted. Adults were maintained at high humidity and 14:10 h light/dark photoperiod.
Single sensillum recordings
Mosquitoes were immobilized by removing wings and legs and were fixed on a glass coverslip covered with double-sided sticky tape. Maxillary palps were extended smoothly onto the glass plate covered with double-sided sticky tape. Chloridized silver wires in drawn-out glass capillaries filled with 0.1% KCl and 0.5% polyvinylpyrrolidone were used as reference and recording electrodes. The reference electrode was placed in the eye, and recording electrode was brought into contact with the sensillum under the microscope (Olympus BX51WI; 800× magnification). The microscope light source was from below, thus allowing a transillumination. Peg sensilla on maxillary palps were clearly visible in transillumination, as compared with direct light from above. Recorded extracellular action potentials (APs) were amplified 1000× and fed into an IDAC4-USB box (Syntech, Hilversum, The Netherlands) via a high-impedance (>1012 Ω) preamplifier and recorded on the hard disk of a PC via a 16-bit analogue–digital IDAC4-USB box and analyzed with the software Auto Spike v. 3.7 (Syntech). AC signals (APs or spikes) were band-pass filtered between 100 and 10 000 Hz, and for the DC signals (receptor potentials/sensillum potentials [SPs]), a high filter of 3 kHz was used. Low-pass filter was set for DC. The activity of colocated ORNs in a sensillum was assessed based on differences in spike amplitude. The ORN with the largest spike amplitude was termed A; the second largest B and the smallest C. Signals were recorded for 10 s (unless otherwise mentioned), starting 2 s before stimulation, and APs were counted off-line in a 500-ms period before stimulation and for 500 ms during stimulation.
Stimulation and stimuli
The preparation was held in a humidified air stream delivered at 20 ml/s via a glass tube (5 mm i.d.) with the outlet placed at approximately 5 mm from the preparation. The needle of the stimuli syringes was introduced from a hole in the tube 40 mm from the outlet. This set-up resulted in a delay of approximately 100 ms due to the travel time of odorant from the stimulus source to the preparation (Syed et al. 2006). The preparation was stimulated with 500-ms pulse during which 1 ml of the synthetic air from a 5-ml polypropylene syringe containing the stimulus was added onto the main air stream. Throughout this study, only synthetic air (ultra zero, CO2 free) was used (Airgas Inc., Sacramento, CA) for the main airflow and stimulus delivery, except for Figure 2 where main airflow was charcoal-filtered room air that contained approximately 350 ppm CO2. To prevent changes in airflow during stimulation, a charcoal-filtered ultra zero, CO2-free airflow of 2 ml/s was delivered via another solenoid valve through a blank syringe into the glass tube, and at the same distance from the preparation, during stimulus off. A gap of at least 1 min or more, following high responses, was allowed between stimulations.
The carbon dioxide stimuli were obtained from 5% CO2/95% O2 medical grade cylinder purchased from Airgas Inc. Different volumes of this mixture were taken into the syringe and diluted to get the desired concentrations. Human breath was blown into the syringe and dilutions were made accordingly. Racemic 1-octen-3-ol and linalool (50:50 as indicated by chiral GC) were from Fluka (St Louis, MO; 98% pure). Most of the carboxylic acids, alcohols, aldehydes, and indoles were purchased from Sigma-Aldrich (St Louis, MO) and were all above 98% pure. DL-lactic acid was 90% pure (Fluka). The 3- and 4-methyl indole were purchased from Acros (Morris Plains, NJ) and were 98% pure. α+β-Thujone was of technical grade (Fluka). Ammonia (25%) was an aqueous solution purchased from Fluka. Optically pure (R)-(−)-1-octen-3-ol, (S)-(+)-1-octen-3-ol, (R)-(+)-1-octyn-3-ol, (S)-(−)-1-octyn-3-ol, (R)-(+)-1-decyn-3-ol, (S)-(−)-1-decyn-3-ol, 1,5-octadiene-3-ol, 1,cis-5-octadiene-3-ol, +/− linalool, D-(+)-linalool, L-(−)-linalool, geraniol, and nerol were kindly provided by Bedoukian Research Inc. (Danbury, CT). Chemicals were dissolved in dichloromethane (DCM), w/v, to make a stock solution of 1 μg/μl and decadic dilutions were made. Ammonia was dissolved in water. An aliquot (10 μl) of a stimulus chemical dissolved in DCM was loaded onto a filter paper strip, the solvent was evaporated for 30 s, and the strip was placed in a 5-ml polypropylene syringe from which various volumes were ejected. DCM alone and an empty syringe served as a control. At least 3 mosquitoes were recorded for each stimulus and up to 3 sensilla were tested from each individual.
It is to be noted that the concentration of CO2 and compounds mentioned throughout this article represent the amount delivered from the point of stimulus release. There was a continuous humidified airflow at 22 ml/s blowing over the mosquito preparation, to which a pulse of the stimulus pulse at 2 ml/s was added resulting in approximately 10× dilution of the stimulus concentration reaching the preparation.
Results
Maxillary palp morphology of C. quinquefasciatus female
Palps were approximately 350 μm long (358 ± 11.8 μm) and had 4 segments (Figure 1a). The first proximal segment was covered with only microtrichia, whereas the rest of the 3 segments were covered with microtrichia, scales, and sensilla chaetica. Club-shaped olfactory basiconic sensilla (variously described earlier as capitate pegs, bulb organs; hence after referred to as peg sensilla) were found only on the fourth segment (Figure 1b). Their density did not vary across the length or over the dorsal or ventral surface. These sensilla measured approximately 16 μm (16.2 ± 1.30 μm) in length, and the tip, a bulbous structure like a club head, was 2.62 ± 0.18 μm wide (Figure 1c). The density was approximately 80 ± 10 sensilla/maxillary palp in females. Scales were most abundant on the dorsal side of the fourth segment.
Scanning electron micrographs of Culex quinquefasciatus head and olfactory appendages. (a) Antennal flagella and maxillary palps (highlighted). (b) Olfactory peg sensilla dispersed among the noninnervated scales and (c) Individual olfactory peg sensilla at higher magnification. Scale bar (a) 250 μm (b) 100 μm (c) 5 μm. Arrows in (b) and asterisks in (c) highlight the sensilla.
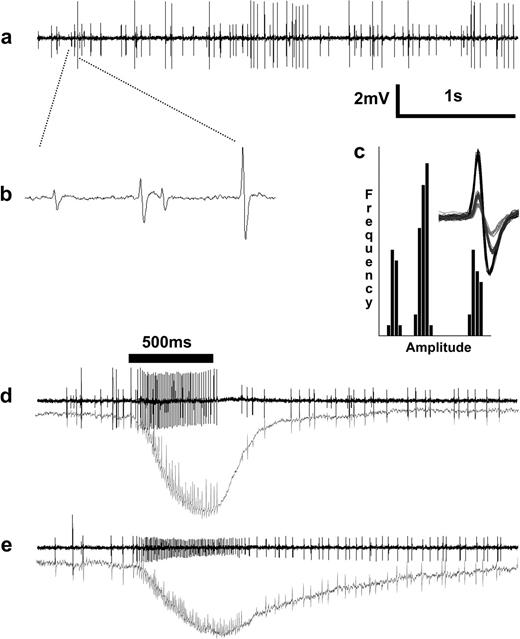
Extracellular single-unit recordings from peg sensilla revealed the presence of 3 ORNs with distinct response characteristics. (a) Spontaneous activity trace from a sensillum showing 3 neurons of specific amplitude and frequency. (b) Expanded trace showing 3 neurons named A, B, and C (high- to low-amplitude, respectively), and (c) amplitude-frequency histograms with an inset showing 3 distinct neuronal spikes clusters. (d) Neuron with largest amplitude (A) responded to CO2 and (e) cell “B” responded to 1-octen-3-ol by increasing the spontaneous firing rate. Lower traces in (d) and (e) are SPs. Main airflow was humidified room air that elicited spontaneous activity from cell A.
Electrophysiology
Extracellularly recorded single-unit activity from peg sensilla revealed APs of 3 different amplitudes, clearly distinguishable from background noise (Figure 2a). We interpreted these spikes as originating from 3 different ORNs that could be clustered into 3 amplitude classes (Figure 2). This interpretation is also supported by the ultrastructural evidence showing 3 ORNs in the palpal peg sensilla in female culicine mosquitoes (McIver 1972). The ORN with the largest spike amplitude was termed A, the second largest B, and the third one C. They showed spontaneous activity in the room air with spike amplitudes approximately 4.0, 2.5, and 1.0 mV, respectively. The amplitudes of B and C cells were variable; sometimes, these 2 ORNs had almost equal spike amplitude and at times cell C had such a small amplitude that it was hidden in the background noise. Cell A showed excitatory responses to increase in CO2, whereas cell B was excited by a very common chemostimuli for hematophagous insects, 1-octen-3-ol (Figure 2d,e). Cell C did not respond to any of the stimuli tested in this study. Excitation of any ORN did not affect the activity of the colocated ORNs, except at very high excitations. A broad screening of common mosquito semiochemicals revealed a rather wide response spectrum for cell B, whrease cell A and C did not respond to any of these tested stimuli (Table 1). Cell B responded best, by increasing firing rate, to many of the compounds of plant origin, namely flower volatiles and common “green leaf volatiles” (GLVs), most of the members of homologous series of straight chain, saturated alcohols, and some low activity to a few aldehydes (Table 1). Cresols, indoles, amines, and carboxylic acids did not elicit any response. Humidity and temperature change did not induce any modulation in spontaneous spiking frequency in any of the 3 ORNs (data not shown).
Response spectra of cell B to various classes of chemicals of animal and plant origin reported as mosquitoes semiochemicals
| Carboxylic acids | |
| Ethanoic acid | ○ |
| Propanoic acid | ○ |
| Butanoic acid | ○ |
| Pentanoic acid | ○ |
| Hexanoic acid | ○ |
| Heptanoic acid | ○ |
| Octanoic acid | ○ |
| Nonanoic acid | ○ |
| Isobutyric acid | ○ |
| Isopentanoic acid | ○ |
| DL-lactic acid | ○ |
| Alcohols | |
| Butanol | + |
| Pentanol | + |
| Hexanol | +++ |
| Heptanol | +++ |
| Octanol | ++ |
| Nonanol | ○ |
| Decanol | ○ |
| 3-Octanol | +++ |
| 1,5-Octadiene-3-ol | ++ |
| Aldehydes | |
| Butanal | ○ |
| Pentanal | + |
| Hexanal | + |
| Heptanal | + |
| Octanal | + |
| Nonanal | ○ |
| Decanal | ○ |
| Esters | |
| Ethyl acetate | ○ |
| Methyl propionate | ○ |
| Methyl butyrate | ○ |
| Ethyl propionate | ○ |
| Ethyl hexanoate | ○ |
| Ethyl-3-hydroxybutyrate | ++ |
| Ethyl-3-hydroxyhexanoate | ++ |
| Ethyl lactate | + |
| Green leaf volatiles | |
| 1-Hexanal | + |
| 1-Hexanol | +++ |
| 1-Hexen-3-ol | +++ |
| (E)-2-Hexen-1-ol | +++ |
| (Z)-2-Hexen-1-ol | ++ |
| (E)-3-Hexen-1-ol | +++ |
| (Z)-3-Hexen-1-ol | ++ |
| (E)-2-Hexenal | ○ |
| (E)-2-Hexenyl acetate | ○ |
| (Z)-3-Hexenyl acetate | ○ |
| Indoles | |
| Indole | ○ |
| 1-Methylindole | ○ |
| 2-Methylindole | ○ |
| 3-Methylindole | ○ |
| 4-Methylindole | ○ |
| 5-Methylindole | ○ |
| 6-Methylindole | ○ |
| 7-Methylindole | ○ |
| Amines | ○ |
| Ammonia | ○ |
| Propylamine | ○ |
| Pentylamine | ○ |
| Hexylamine | ○ |
| Cresols | |
| 2-Methylphenol | ○ |
| 3-Methylphenol | ○ |
| 4-Methylphenol | ○ |
| Floral volatiles | |
| Limonene | ○ |
| β-Myrcene | + |
| Racemic linalool | +++ |
| α-Pinene | + |
| Benzaldehyde | ○ |
| β-Pinene | + |
| β-Caryophyllene | + |
| 6-Methyl-5-heptene-2-one | + |
| α+β-Thujone | +++ |
| Carboxylic acids | |
| Ethanoic acid | ○ |
| Propanoic acid | ○ |
| Butanoic acid | ○ |
| Pentanoic acid | ○ |
| Hexanoic acid | ○ |
| Heptanoic acid | ○ |
| Octanoic acid | ○ |
| Nonanoic acid | ○ |
| Isobutyric acid | ○ |
| Isopentanoic acid | ○ |
| DL-lactic acid | ○ |
| Alcohols | |
| Butanol | + |
| Pentanol | + |
| Hexanol | +++ |
| Heptanol | +++ |
| Octanol | ++ |
| Nonanol | ○ |
| Decanol | ○ |
| 3-Octanol | +++ |
| 1,5-Octadiene-3-ol | ++ |
| Aldehydes | |
| Butanal | ○ |
| Pentanal | + |
| Hexanal | + |
| Heptanal | + |
| Octanal | + |
| Nonanal | ○ |
| Decanal | ○ |
| Esters | |
| Ethyl acetate | ○ |
| Methyl propionate | ○ |
| Methyl butyrate | ○ |
| Ethyl propionate | ○ |
| Ethyl hexanoate | ○ |
| Ethyl-3-hydroxybutyrate | ++ |
| Ethyl-3-hydroxyhexanoate | ++ |
| Ethyl lactate | + |
| Green leaf volatiles | |
| 1-Hexanal | + |
| 1-Hexanol | +++ |
| 1-Hexen-3-ol | +++ |
| (E)-2-Hexen-1-ol | +++ |
| (Z)-2-Hexen-1-ol | ++ |
| (E)-3-Hexen-1-ol | +++ |
| (Z)-3-Hexen-1-ol | ++ |
| (E)-2-Hexenal | ○ |
| (E)-2-Hexenyl acetate | ○ |
| (Z)-3-Hexenyl acetate | ○ |
| Indoles | |
| Indole | ○ |
| 1-Methylindole | ○ |
| 2-Methylindole | ○ |
| 3-Methylindole | ○ |
| 4-Methylindole | ○ |
| 5-Methylindole | ○ |
| 6-Methylindole | ○ |
| 7-Methylindole | ○ |
| Amines | ○ |
| Ammonia | ○ |
| Propylamine | ○ |
| Pentylamine | ○ |
| Hexylamine | ○ |
| Cresols | |
| 2-Methylphenol | ○ |
| 3-Methylphenol | ○ |
| 4-Methylphenol | ○ |
| Floral volatiles | |
| Limonene | ○ |
| β-Myrcene | + |
| Racemic linalool | +++ |
| α-Pinene | + |
| Benzaldehyde | ○ |
| β-Pinene | + |
| β-Caryophyllene | + |
| 6-Methyl-5-heptene-2-one | + |
| α+β-Thujone | +++ |
○, no response; +, up to 25% increase; ++, 25–50% increase; and +++, 50–100% increase. Percent increase is the relative percent increase of the excitation of the cell “B” (202 spikes/s) as in response to racemic 1-octen-3-ol at 100 ng dose. All compounds were tested at 10 μg in DCM, except ammonia that was dissolved in water and tested at approximately 0.05%. Racemic 1-octen-3-ol was tested at 100 ng and used as standard to calculate the relative excitation levels of other compounds (n = 5–17).
Response spectra of cell B to various classes of chemicals of animal and plant origin reported as mosquitoes semiochemicals
| Carboxylic acids | |
| Ethanoic acid | ○ |
| Propanoic acid | ○ |
| Butanoic acid | ○ |
| Pentanoic acid | ○ |
| Hexanoic acid | ○ |
| Heptanoic acid | ○ |
| Octanoic acid | ○ |
| Nonanoic acid | ○ |
| Isobutyric acid | ○ |
| Isopentanoic acid | ○ |
| DL-lactic acid | ○ |
| Alcohols | |
| Butanol | + |
| Pentanol | + |
| Hexanol | +++ |
| Heptanol | +++ |
| Octanol | ++ |
| Nonanol | ○ |
| Decanol | ○ |
| 3-Octanol | +++ |
| 1,5-Octadiene-3-ol | ++ |
| Aldehydes | |
| Butanal | ○ |
| Pentanal | + |
| Hexanal | + |
| Heptanal | + |
| Octanal | + |
| Nonanal | ○ |
| Decanal | ○ |
| Esters | |
| Ethyl acetate | ○ |
| Methyl propionate | ○ |
| Methyl butyrate | ○ |
| Ethyl propionate | ○ |
| Ethyl hexanoate | ○ |
| Ethyl-3-hydroxybutyrate | ++ |
| Ethyl-3-hydroxyhexanoate | ++ |
| Ethyl lactate | + |
| Green leaf volatiles | |
| 1-Hexanal | + |
| 1-Hexanol | +++ |
| 1-Hexen-3-ol | +++ |
| (E)-2-Hexen-1-ol | +++ |
| (Z)-2-Hexen-1-ol | ++ |
| (E)-3-Hexen-1-ol | +++ |
| (Z)-3-Hexen-1-ol | ++ |
| (E)-2-Hexenal | ○ |
| (E)-2-Hexenyl acetate | ○ |
| (Z)-3-Hexenyl acetate | ○ |
| Indoles | |
| Indole | ○ |
| 1-Methylindole | ○ |
| 2-Methylindole | ○ |
| 3-Methylindole | ○ |
| 4-Methylindole | ○ |
| 5-Methylindole | ○ |
| 6-Methylindole | ○ |
| 7-Methylindole | ○ |
| Amines | ○ |
| Ammonia | ○ |
| Propylamine | ○ |
| Pentylamine | ○ |
| Hexylamine | ○ |
| Cresols | |
| 2-Methylphenol | ○ |
| 3-Methylphenol | ○ |
| 4-Methylphenol | ○ |
| Floral volatiles | |
| Limonene | ○ |
| β-Myrcene | + |
| Racemic linalool | +++ |
| α-Pinene | + |
| Benzaldehyde | ○ |
| β-Pinene | + |
| β-Caryophyllene | + |
| 6-Methyl-5-heptene-2-one | + |
| α+β-Thujone | +++ |
| Carboxylic acids | |
| Ethanoic acid | ○ |
| Propanoic acid | ○ |
| Butanoic acid | ○ |
| Pentanoic acid | ○ |
| Hexanoic acid | ○ |
| Heptanoic acid | ○ |
| Octanoic acid | ○ |
| Nonanoic acid | ○ |
| Isobutyric acid | ○ |
| Isopentanoic acid | ○ |
| DL-lactic acid | ○ |
| Alcohols | |
| Butanol | + |
| Pentanol | + |
| Hexanol | +++ |
| Heptanol | +++ |
| Octanol | ++ |
| Nonanol | ○ |
| Decanol | ○ |
| 3-Octanol | +++ |
| 1,5-Octadiene-3-ol | ++ |
| Aldehydes | |
| Butanal | ○ |
| Pentanal | + |
| Hexanal | + |
| Heptanal | + |
| Octanal | + |
| Nonanal | ○ |
| Decanal | ○ |
| Esters | |
| Ethyl acetate | ○ |
| Methyl propionate | ○ |
| Methyl butyrate | ○ |
| Ethyl propionate | ○ |
| Ethyl hexanoate | ○ |
| Ethyl-3-hydroxybutyrate | ++ |
| Ethyl-3-hydroxyhexanoate | ++ |
| Ethyl lactate | + |
| Green leaf volatiles | |
| 1-Hexanal | + |
| 1-Hexanol | +++ |
| 1-Hexen-3-ol | +++ |
| (E)-2-Hexen-1-ol | +++ |
| (Z)-2-Hexen-1-ol | ++ |
| (E)-3-Hexen-1-ol | +++ |
| (Z)-3-Hexen-1-ol | ++ |
| (E)-2-Hexenal | ○ |
| (E)-2-Hexenyl acetate | ○ |
| (Z)-3-Hexenyl acetate | ○ |
| Indoles | |
| Indole | ○ |
| 1-Methylindole | ○ |
| 2-Methylindole | ○ |
| 3-Methylindole | ○ |
| 4-Methylindole | ○ |
| 5-Methylindole | ○ |
| 6-Methylindole | ○ |
| 7-Methylindole | ○ |
| Amines | ○ |
| Ammonia | ○ |
| Propylamine | ○ |
| Pentylamine | ○ |
| Hexylamine | ○ |
| Cresols | |
| 2-Methylphenol | ○ |
| 3-Methylphenol | ○ |
| 4-Methylphenol | ○ |
| Floral volatiles | |
| Limonene | ○ |
| β-Myrcene | + |
| Racemic linalool | +++ |
| α-Pinene | + |
| Benzaldehyde | ○ |
| β-Pinene | + |
| β-Caryophyllene | + |
| 6-Methyl-5-heptene-2-one | + |
| α+β-Thujone | +++ |
○, no response; +, up to 25% increase; ++, 25–50% increase; and +++, 50–100% increase. Percent increase is the relative percent increase of the excitation of the cell “B” (202 spikes/s) as in response to racemic 1-octen-3-ol at 100 ng dose. All compounds were tested at 10 μg in DCM, except ammonia that was dissolved in water and tested at approximately 0.05%. Racemic 1-octen-3-ol was tested at 100 ng and used as standard to calculate the relative excitation levels of other compounds (n = 5–17).
CO2 reception by cell A
Concentration-dependent responses
Carbon dioxide was detected by cell A in a concentration-dependent manner (Figure 3a–c). An addition of 0.24% CO2 to the main airflow elicited significant response with characteristic dynamics. Of the 15 sensilla recorded, 4 sensilla did not respond to the 0.24% augmentation. All sensilla, however, responded to 0.4% or higher doses. A sharp response onset upon stimulation and a quick termination was detected across the range of concentrations tested. Human breath elicited excitatory dose-dependent responses from cell A (Figure 3b, inset) that were comparable to the extrapolated levels of CO2 present in human breath (ca. 4%). There was no change in cell B activity upon breath stimulation. Sharp spiking onset and offset in response to CO2 was mirrored in SP responses recorded from the sensilla (Figure 3c). Step increase in CO2 concentrations caused sharper onset of responses (Figure 3c, inset), but the overall rise and fall pattern simply followed the stimulus delivery period. It is worth mentioning that CO2 response pattern was remarkably different from cell B responses (see below).
CO2 elicited concentration-dependent excitatory responses from cell A in peg sensilla. (a) AP traces showing responses to increasing concentrations of CO2 (top to bottom). (b) Concentration-dependent curve of CO2 responses from cell A (n = 15; mean ± standard error of the mean [SEM]). Inset shows response of different dilutions of breath (n = 8; mean ± SEM). (c) SPs indicate the magnitude and kinetics (expanded in inset) of response. Note that the CO2 concentrations indicated here are those added onto the main airflow that is CO2-free synthetic air, resulting in approximately 10× dilution. Thus, a 0.4% value here indicates approximately 400 ppm CO2 reaching the sensillum.
CO2 response kinetics
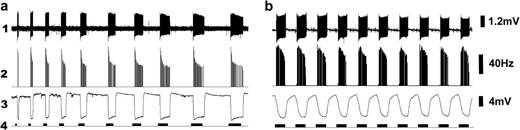
CO2 induced a phasic-tonic response from cell A that could be detected both in the spiking pattern and the SP dynamics. Stimulations with 2% CO2 (a dose in the middle of the dynamic range of dose-response curve) pulses ranging from 1–10 s length revealed a consistent pattern of spiking dynamics where a sharp phasic response onset is followed by the sustained tonic activity that remained unaltered independent of the stimulation length (Figure 4a). Cell A did not adapt to repeated stimulation of 1 s (Figure 4b).
CO2-sensitive neuron in peg sensilla respond to augmentation of CO2 in phasic-tonic mode, independent of the stimulation length and frequency. Sensilla were stimulated with approximately 2% CO2 added onto a CO2-free main airflow. (a) In a step increase of CO2 stimulus duration (1 s to 10 s, from left to right), cell A responded with a sharp phasic mode followed by a sustained tonic phase that remained independent of the stimulus duration. (b) One second of stimulation followed by 1-s interval produces a quick rebound and the spiking pattern remained phasic tonic. In both experiments, (1) shows spike trace, (2) rate-time histogram obtained by extracting the cell A spikes and 50 ms binned, (3) SPs faithfully representing the spiking pattern, with a sharp response onset and offset following stimulation, and (4) stimulation protocol. Responses were reproducible.
Sensitivity and selectivity of cell B
1-octen-3-ol and related compounds
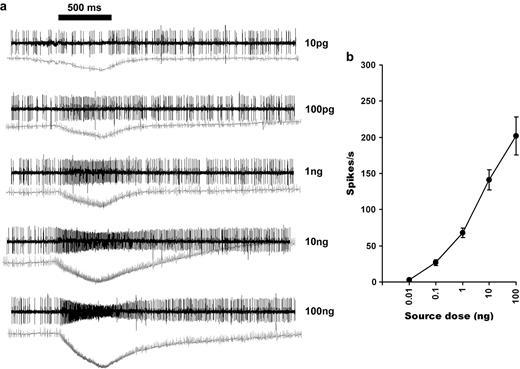
Racemic 1-octen-3-ol elicited dose-dependent responses from cell B with a response threshold of 100 pg (Figure 5a,b). Further screening of 2 structurally related compounds revealed a comparable sensitivity to racemic 1-octyn-3-ol, whereas response to the saturated 8-carbon alcohol, 3-octanol, was of 10-fold lower (Figure 6a). Cell B also showed a remarkable selectivity between 2 enantiomers of 1-octen-3-ol (Figure 6b), with (R)-(−)-1-octen-3-ol eliciting a robust responses at 10 ng dose (256.6 ± 12 spikes/s), whereas its antipode, S-(+)-1-octen-3-ol, did not elicit any significant responses at that dose. S-(+)-1-octen-3-ol elicited activity only at higher doses, with 100 ng eliciting 39.7 ± 10 spikes/s, and the highest tested dose, 1 μg eliciting 115.5 ± 23 spikes/s. Solvent alone generated 2 ± 1 spikes/s. Racemic 1-octen-3-ol and 1-octyn-3-ol elicited approximately half the frequency of that elicited by the corresponding (R)-(−) enantiomer at 10 ng dose (Figure 7). A related diene alcohol, 1,5-octadien-3-ol, induced excitation in cell B with (R)-1-(Z)-5- enantiomer inducing significantly higher responses (Figure 7).
Peg sensilla house an ORN that responds with high sensitive to 1-octen-3-ol by increasing the firing rate. (a) AP traces of the cell B to the increasing dose of racemic 1-octen-3-ol. Lower traces are SPs. (b) Dose-response curve (n = 22; mean ± standard error of the mean).
1-octen-3-ol–sensitive ORN does not differentiate structural homologues but shows a remarkable enantiomeric specificity. (a) 1-octen-3-ol and 1-octyn-3-ol elicited comparable responses, though the responses from 1-octen-3-ol were consistently higher (n = 22; mean ± standard error of the mean [SEM] for 1-octen-3-ol and n = 18; mean ± SEM for 1-octyn-3-ol). The 3-octanol responses are slightly lower (n = 5; mean ± SEM) (b) the natural enantiomer, (R)-(−)-1-octen-3-ol, elicited significantly higher responses as compared with its antipod, (S)-(+)-1-octen-3-ol (n = 10; mean ± SEM).
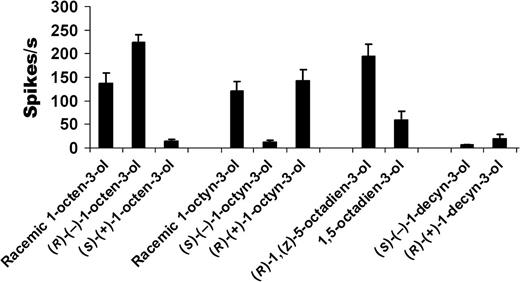
Enantiomeric selectivity of cell B to racemates and individual enantiomers of 1-octen-3-ol and related compounds. R enantiomers elicited higher responses than S forms. All compounds were tested at 10 ng source dose (n = 5; mean ± standard error of the mean).
Linalool and “green leaf volatiles”
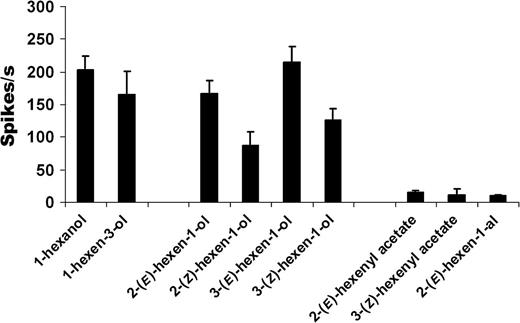
Linalool and one of its structural homologue, dehydrolinalool, elicited dose-dependent and comparable responses from cell B (Figure 8a). Linalool-induced excitatory responses were again optically selective, with L-(−) isomer inducing higher responses than D-(+)-linalool (Figure 8b). Responses to racemic linalool (Figure 8a) were higher compared with enantiomers (Figure 8b) as the tests were conducted on different animals. Alcoholic green leaf volatiles induced increase in spike rates, albeit low sensitivity. At 10 μg dose, (E) isomers of 2- and 3-hexen-1-ol elicited responses that were almost double the responses of their (Z)-counterparts (Figure 9). Green leaf esters and aldehydes did not induce any response (Table 1).
Linalool-induced excitatory responses from cell B; (a) Racemic linalool and one of its structural homologue, dehydrolinalool, elicited comparable responses (n = 5; mean ± standard error of the mean [SEM]); (b)L-(−)-Linalool elicited higher responses than its D-(+) enantiomer (n = 9; mean ± SEM).
Responses of cell B to common green leaf volatiles and isomeric selectivity. Most of the common green leaf volatiles with alcohol moiety elicited responses from cell B with the (E) isomers generating higher responses than the (Z) counterparts (n = 5; mean ± standard error of the mean). All compounds were tested at 10 μg source dose.
Discussion
Two of the most widely used chemostimuli in mosquito research, carbon dioxide and 1-octen-3-ol, are detected by ORNs located in the peg sensilla on maxillary palps in C. quinquefasciatus. Another surprising finding in this study was that the second ORN responds to a variety of compounds, particularly plant-derived semiochemicals. A detailed electrophysiological study from peg sensilla on maxillary palps of A. aegypti identified an ORN extremely sensitive to CO2, with a response threshold of approximately 300 ppm (Grant et al. 1995). These authors also did limited recordings from C. quinquefasciatus showing similar threshold. Our results corroborate those preliminary findings and extend the understanding to dose-response function and response kinetics of this important chemostimuli for C. quinquefasciatus. We measured CO2 detection threshold in cell A to be approximately 240 ppm (reaching the sensillum), and we also tested up to 4% CO2 (ca. human breath concentrations) at the source dose and demonstrate that cell A can still detect those concentrations reliably. Our studies were conducted with glass electrodes that allowed us to record action and SPs simultaneously. In our single-cell recordings, electrical events in ORNs can be measured from the sensillum lymph, upon penetration, as extracellular potentials, both in the form of APs generated by the neurons and SP, which most probably reflect the receptor potentials of the individual neurons (Stengl et al. 1992; Kaissling et al. 1995). In Drosophila, a strong correlation between SPs and APs has been found, and moreover, SPs faithfully represented the spiking dynamics (Syed et al. 2004). In ambient air, cell A was spontaneously active (Figure 2), whereas these cells remained silent in the CO2 free air (Figures 3 on). CO2 responses from cell A had a threshold of approximately 240 ppm (Figure 3), which is lower than the ambient atmospheric CO2 concentration, approximately 350 ppm. CO2 responses were tonic at the lower concentrations but remained phasic tonic over a wide concentration range.
Pure human breath elicited 164 ± 8 spikes/s from CO2-sensitive ORN, as compared with 168.8 ± 7 spikes/s upon stimulation with 3.2% CO2. Dilutions of breath elicited 111 ± 18 and 46.5 ± 14 spikes/s for 2× and 4× dilution, respectively, suggesting that breath contained approximately 3.5% CO2 (Figure 2, inset). SP amplitude and kinetics (Figure 3c) faithfully represented the spiking pattern of the ORN (Figure 3a). The response onset was sharper with lower latency at successive stimulations with increasing CO2 concentrations. Another characteristic feature of the CO2-sensitive ORN was the absence of adaptation to prolong and repeated stimulations with CO2. Short-term adaptation, reversible reduction of sensitivity due to prior stimulation (Dolzer et al. 2003), and desensitization, decline in sensitivity as seen due to a stimulus of long duration (Zufall and Leinders-Zufall 2000), are seen in various ORNs, especially, in pheromone-sensitive moth ORNs (Kaissling et al. 1986). Absence of these 2 kinds of adaptation in CO2-sensitive ORNs (Figure 4) underlines the physiological role of these ORNs in the ecological context of CO2. Nonadapting CO2-sensitive ORNs would offer an advantage in this situation, as they are available to any random bursts of any length and strength, and that they act as absolute detectors, in that background concentrations have no influence on their ability to respond to step increase in CO2 concentration (Grant et al. 1995). In wind tunnel experiments, a brief exposure of a CO2 pulse enhances the sensitivity of mosquitoes to “human hand odors” by at least 5-folds (Dekker et al. 2005). The importance of CO2 as a potential source of host/habitat cue in the sensory ecology of mosquitoes is highlighted by the fact that CO2 is the only odorant that consistently increased capture rates of many mosquito species (Mboera and Takken 1997); peg sensilla are found in both sexes of culicine (McIver and Charlton 1970; McIver 1971) and anopheline mosquitoes (McIver and Siemicki 1975) and that CO2-sensitive ORNs exhibit comparable sensitivity across many species (Grant et al. 1995). In a limited set of recordings, we measured similar sensitivity and CO2 response dynamics from ORNs in palpal peg sensilla in male C. quinquefasciatus (data not shown).
In Drosophila, CO2-sensitive ORNs are located in one of the basiconic sensillum on antenna (de Bruyne et al. 2001). A gustatory receptor, Gr21a, is shown to be involved in CO2 reception, the population of ORNs expressing Gr21a in these sensilla are demonstrated to govern the characteristic avoidance behavior in adult Drosophila, and the CO2-detecting ORNs converged onto a distinct glomerulus in antennal lobe (Suh et al. 2004). Role of Gr21a has been further explored showing that another Gr, Gr63a, is necessary to confer electrophysiological (Jones et al. 2007; Kwon et al. 2007) and behavioral responses to CO2 (Jones et al. 2007). Both these studies showed that the ectopic expression of Gr21a and Gr63a together confers CO2 sensitivity in CO2-insensitive olfactory neurons, thus convincingly demonstrating that these Grs indeed govern CO2 reception. Interestingly, Jones et al. have also identified mosquito homologues of Gr21a and Gr63a in A. gambiae, GPRGR22 and GPRGR24, and show their coexpression in an ORN on A. gambiae maxillary palps (Jones et al. 2007).
Racemic 1-octen-3-ol elicited excitatory responses from cell B at a dose of 100 pg, rivaling the sensitivity of many pheromone receptors neurons (Kaissling 1987). This compound was originally identified during research for attractants for tsetse flies, Glossina (Hall et al. 1984), but has since been shown to act as kairomone, alone and in combination with other compounds, in various hematophagous arthropods: C. quinquefasciatus (Mboera et al. 2000) and many other mosquito species (Clements 1999); bont tick, Amblyomma variegatum (McMahon et al. 2001); and a hematophagous bug, Triatoma infestans (Barrozo and Lazzari 2004). 1-Octen-3-ol was also identified from human sweat and showed a dose-dependent response in antennogram studies in A. gambiae (Cork and Park 1996). In our study, we did not find any change in the activity of 1-octen-3-ol–sensitive ORN (cell B) upon stimulation with pure breath, indicating that there is no 1-octen-3-ol present, at least within the detection threshold of cell B, in the human breath. Interestingly, the ORN responding to 1-octen-3-ol were insensitive to a variety of chemostimuli identified from hosts as mosquito kairomones, like carboxylic acids, amines, skatoles, and indoles (Cork and Park 1996; Meijerink et al. 2001). 1-Octen-3-ol is widely cited as component of cow breath, but unambiguous evidence is still lacking. This alcohol was originally identified in odors collected from a room that enclosed a cow with fodder (Hall et al. 1984). Although 1-octen-3-ol was not collected from the room with fodder alone, this does not preclude the possibility that 1-octen-3-ol was product of rumination and/or skin odors. 1-Octen-3-ol has also been identified as a component in many floral odors (Knudsen et al. 1993). An alkyl form of 1-octen-3-ol elicited comparable dose-dependent responses from cell B, and response to a saturated 3-octanol was one order of magnitude lower, indicating that unsaturation at the first carbon is critical. In tsetse flies, single sensillum recordings studies from a population of 1-octen-3-ol–sensitive ORNs (van Naters et al. 1996) and antennal movement responses study (Saini et al. 1989) essentially gave similar results, indicating 1-octyn-3-ol to be equally effective as 1-octen-3-ol and 3-octanol with a lower potency. Enantiomeric composition of 1-octen-3-ol was determined as 80:20 to 92:8 R:S in different collections, but no enantiomeric differences were observed either in electroantennogram measurement or behavioral studies in a wind tunnel and field (Hall et al. 1984). Behavioral responses induced by racemate or (R)-(−) enantiomer were not significantly different in A. variegatum, both increasing upwind walk on a servosphere (McMahon et al. 2001). Lantana camara, an invasive plant in many sub-Saharan African countries, was reported as attracting tsetse flies in a wind tunnel, and further identification of active components revealed 1-octen-3-ol as one of the main active components, with a racemic composition approximately 95:5 R:S (Syed 2002; Syed and Guerin 2004). The enantiomeric selectivity of receptors in cell B in peg sensilla here is remarkable, with (R)-(−) enantiomer, eliciting extremely high response (267 ± 5.7 spikes/s) at a source dose of 10 ng (Figure 7b). Various enantiomers of 1-octen-3-ol–related compounds again elicited selective responses (Figure 8).
Linalool is a common floral scent constituent, present in 70% of the families of the seed plants studied (Knudsen et al. 2006). Linalool and dehydrolinalool were suggested to act as attractants or spatial repellents for A. aegypti based on the observation that when used alone, linalool attracts mosquitoes to a trap, but when used with CO2 or with CO2 + octenol, linalool reduced mosquito collection sizes by as much as 50%, and linalool reduced the number of active mosquitoes in a population in response to an attractive mixture (Kline et al. 2003). We report here that both linalool and dehydrolinalool are indeed detected by an ORN in palps peg sensilla and that the L-(−) isomer elicits higher responses than D-(+)-linalool (Figure 8), though the sensitivity was much lower than that of 1-octen-3-ol. All GLVs with alcohol moiety tested elicited varying responses, with (E) isomers eliciting significantly higher responses than (Z)-forms (Figure 9). GLVs are 6-carbon alcohols, aldehydes, and their ester derivatives, which are especially abundant in herbaceous plants, angiosperm shrubs, and trees. They are produced by oxidative degradation of leaf lipids and continuously released by leaves (Visser and Ave 1978; Visser 1986). This study is in line with the observations in tsetse flies, Glossina spp., that many terpenes, GLVs, and aromatics isolated from an “attractive” plant, L. camara, elicited strong electrophysiological responses from antennal chemoreceptors. Even though tsetse flies of both sexes do not feed on plants at any point in life, these plant-derived compounds induced upwind anemotactic responses in the wind tunnel (Syed and Guerin 2004). In this case, it was argued that the habitat location is vital in the very survival of tsetse flies and that these receptors might act in combinatorial way in context-dependent manner, allowing them to locate host and/or habitats, based upon the nutritional state of flies (Syed and Guerin 2004).
Maxillary palps in C. quinquefasciatus with a single type of sensilla population housing 2 ORNs dedicated to detection of CO2 and plant-related compounds suggest a rather elaborate role for maxillary palps in mosquito biology than previously envisioned. We propose that maxillary palps are also important detectors for plant and nectar sources, with dedicated ORNs for CO2, GLVs, and floral compounds. Preliminary data indicated that CO2 and 1-octen-3-ol–sensitive ORNs in peg sensilla from male maxillary palp responded to these 2 chemostimuli in a dose-dependent manner and with comparable sensitivity to female ORNs (data not shown). Many adult mosquitoes have to find and feed on nectar for flight energy and survival (Foster 1995); thus, it is conceivable that they developed a sophisticated detection system. Attraction to various flower extracts has already been shown in Anopheles arabiensis (Healy and Jepson 1988) and Culex pipiens pipiens (Mauer and Rowley 1999). A unique role of nectar-related odors has been reported in A. gambiae, wherein both males and females showed stronger responses to nectar-related odors than human odors soon after emergence and that this sensitivity was maintained throughout the male's life and that females gradually (5 days on) develop preference for human odors (Foster and Takken 2004). In a detailed study on possible plant preferences of A. gambiae for nectar under semifield conditions, it has been shown that males and females of A. gambiae forage and survive on naturally occurring plant substances (Impoinvil et al. 2004). A direct analogy of physiology and role of maxillary palp organ in mosquitoes responding to CO2 and nectar/plant-related volatiles was observed in hawk moth, Manduca sexta. A specialized organ, labial pit organ, in labial palps has dedicated ORNs for CO2 detection that converge onto a specialized glomerulus in the antennal lobe (Guerenstein et al. 2004). Adult hawk moths were shown to seek and prefer surrogate flowers that were scented with flower essential oils and raised CO2 levels over flowers without elevated CO2 (Thom et al. 2004).
Although we still have to test the hypothesis put forward in this article under various behavioral paradigms, we cannot exclude the possibility that mosquitoes identify and track nectar-rich flowers based on olfactory detection and discrimination of volatile profile of plant and/or flowers. These semiochemicals alone or in combination with CO2 may pave the way for the development of novel attractants for trapping both male and female Culex mosquitoes.
This work was supported in part by National Institutes of Health grant (1U01AI05826-01) and a research agreement between Bedoukian Research Inc. and University of California (UC), Davis. We thank Bedoukian Research Inc. for providing optically pure compounds and Dr Anthony Cornel and Julie Christensen of UC Davis for providing mosquitoes.
References
Author notes
Dedicated to Dr Jan van der Pers (1944–2006).



![CO2 elicited concentration-dependent excitatory responses from cell A in peg sensilla. (a) AP traces showing responses to increasing concentrations of CO2 (top to bottom). (b) Concentration-dependent curve of CO2 responses from cell A (n = 15; mean ± standard error of the mean [SEM]). Inset shows response of different dilutions of breath (n = 8; mean ± SEM). (c) SPs indicate the magnitude and kinetics (expanded in inset) of response. Note that the CO2 concentrations indicated here are those added onto the main airflow that is CO2-free synthetic air, resulting in approximately 10× dilution. Thus, a 0.4% value here indicates approximately 400 ppm CO2 reaching the sensillum.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/32/8/10.1093/chemse/bjm040/2/m_chemsebjm040f03_ht.jpeg?Expires=1716368803&Signature=FRbmHbSG9utHWW4j2PKSOnNhOgeF2edZafE9oiMLZ9RbmdLnOdwAdRkJEQuLcWgpiB9hWKG-qThYKSM0LhP1paxNpWJZ4u7yQVYldNemoWUZtuWlNNMRJXq694RaibYvLZBXTirieDSkwVHxDWxiDGLYDTVythXQsC4ktWbC7ltolGILtC280n82M6rFxv6ruASdx2RqHiddfXlwpLDwexw4uF5Dvkjqa6XfMdaec-tQffbk8Reoj8u-vnmrIVuvL1RezSPI~vnnK9HjVnIEKgejcU5V~65FAM8YWOBIIBzNJlbRMo8Zz7NGY-2s4Pkn2Gn-hqvOlikONpk~kgHdyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![1-octen-3-ol–sensitive ORN does not differentiate structural homologues but shows a remarkable enantiomeric specificity. (a) 1-octen-3-ol and 1-octyn-3-ol elicited comparable responses, though the responses from 1-octen-3-ol were consistently higher (n = 22; mean ± standard error of the mean [SEM] for 1-octen-3-ol and n = 18; mean ± SEM for 1-octyn-3-ol). The 3-octanol responses are slightly lower (n = 5; mean ± SEM) (b) the natural enantiomer, (R)-(−)-1-octen-3-ol, elicited significantly higher responses as compared with its antipod, (S)-(+)-1-octen-3-ol (n = 10; mean ± SEM).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/32/8/10.1093/chemse/bjm040/2/m_chemsebjm040f06_lw.jpeg?Expires=1716368803&Signature=q~SPBughZ6HX6Epc9AYdbOIccaN8wKEyrSH5CG5rPOpVL94JeYw6AAQ42bc2Svq8ClS7eoJNKhG5F~vKCuMVPgDmf8J4AiFrLB5j8UG8SwyzJ9PsDYbTxiPmhNfsreoP6TKJxalDI6H~OzKCgab2dKX~M9s5wwdrg338P3vozP-mhSykzqrTx7y-QQewzkXJFaWkxez~oPVPKLF7OhhST8TxndhVLlJvNiUg8wMdHz~y-DZlcnbk5nPrX7TK9n0NsWM2OutqcN4HNAY~Pu-VRz8BAcWi9e-r~2G-RXSICZ5DW6IYRSM9Rb9vXMUQRGuh1KtaWZF-1KzeLZcNLe2A5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Linalool-induced excitatory responses from cell B; (a) Racemic linalool and one of its structural homologue, dehydrolinalool, elicited comparable responses (n = 5; mean ± standard error of the mean [SEM]); (b)L-(−)-Linalool elicited higher responses than its D-(+) enantiomer (n = 9; mean ± SEM).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/chemse/32/8/10.1093/chemse/bjm040/2/m_chemsebjm040f08_lw.jpeg?Expires=1716368803&Signature=dArZWSsMLA-52ePXjPudLqDYaWS199QtepGxuS0qV8PIPfDMuAkLp6TO6bl1~kvLjwwJK-eiFIZFDVaqA~MruwZsEO8SX3YBIhJUbd-Uth4n93vylqmTSJK6FQ2AwtEUaWoAgZCLQJ3O6LeQoK3GparuBg5gZtQmduZUbUNlpQ6s6tzOayUtHvWopTMswXX4AOSzk2wYhGUaGPmL8Wr8Rzr7xJ~yjUu5vd8wpfJi8ThTXfwuZ7HUdgXBKhSGxUHPlq4e84yszNiCVIDOHZFZg6KrIfBa8pvknwSU8eA5j0p3JFnJkTevtLQQF62OhvmKCqvsZNgggnmX-y141HcNjw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)