Qualitative assessment of the risk to the UK human population of monkeypox infection in a canine, feline, mustelid, lagomorph or rodent UK pet
Updated 30 August 2022
About the Human Animal Infections and Risk Surveillance group
This document was prepared by the UK Health Security Agency (UKHSA) on behalf of the joint Human Animal Infections and Risk Surveillance (HAIRS) group.
HAIRS is a multi-agency cross-government horizon scanning and risk assessment group, which acts as a forum to identify and discuss infections with potential for interspecies transfer (particularly zoonotic infections).
Members include representatives from the UKHSA, Department for the Environment, Food and Rural Affairs (Defra), Department of Health and Social Care (DHSC), Animal and Plant Health Agency, Food Standards Agency, Public Health Wales, Welsh Government, Public Health Scotland, Scottish Government, Public Health Agency of Northern Ireland and the Department of Agriculture, Environment and Rural Affairs for Northern Ireland.
Information on the risk assessment processes used by the HAIRS group can be found at HAIRS risk assessment process.

Version control
Date of this assessment: 22 May 2022
Version: 2.0
Reason for the assessment: Reports of human-to-human transmission of monkeypox virus in the UK; concerns for pets in the homes of infected people.
Reason for update: Reflect the change in monkeypox clade nomenclature and acknowledge reports of human-to-dog transmission of monkeypox virus within households in Brazil and France.
Completed by: HAIRS members.
Non-HAIRS group experts consulted: Dr. Lorraine McElhinney, Dr. Pip Beard.
Date of initial risk assessment: 22 May 2022
Information on the risk assessment processes used by the HAIRS group can be found online.
Summary
In May 2022, human-to-human transmission of monkeypox virus was observed in several non-endemic countries, including the UK.
During a previous monkeypox incident in the UK in 2018, a pet management process had to be swiftly implemented for one affected household, without conducting a risk assessment a priori.
As the numbers of affected households in the UK related to the current outbreak are rapidly increasing, this warrants a more thorough assessment of the risk posed by mammalian pets exposed to monkeypox virus to people with whom they may subsequently come into contact.
For the purposes of the assessment, it is presumed the pet is present in the contaminated household of a confirmed human monkeypox case. The risk posed is therefore to the non-infected human contacts, other domestic animals, or in-contact peridomestic or wild rodents. It is concluded that the highest risk is posed by the presence of pet rodents, more so than lagomorphs, canids, felids and mustelids.
It is unlikely (but cannot be ruled out) that an infected rodent pet could spread infection to peridomestic or wild rodents. As rodents may not show clinical signs of infection, and the incubation period is unknown, testing to detect the presence of antibodies as well as virus would provide more confidence in ruling out infection.
The evidence of susceptibility for non-rodent pets is poor or incomplete, and therefore a precautionary risk management process should be considered.
Based on current evidence, for pet rodents of susceptible species in households where there are infected people, temporary removal from the household for a limited quarantine period (21 days) and testing to exclude infection is recommended, particularly where there are infected human contacts who have had close direct and prolonged contact with the animal or its bedding and/or litter.
Appropriate risk management for laboratory staff handling samples, or vets and animal health professionals handling or taking samples from the pets, should also be established.
Assessment of the risk of infection to the human population in the UK
Probability: general population – very low.
The probability of infection would be considered moderate to high for individuals interacting with infected pets (notably rodents) and their contaminated environments.
Impact: very low to low.
Level of confidence in assessment of risk
Satisfactory.
Evidence is incomplete for the susceptibility of endemic UK rodent populations to monkeypox infection, but there is evidence for non-native rodent susceptibility; limited or no evidence for susceptibility of non-rodent mammalian pets. Some evidence from previous outbreaks of animal-to-animal transmission or rodent-to-human transmission.
Action(s) and/or recommendations
Remove rodent pets of susceptible species from a household to secure accommodation, isolate for Category 3 pathogen, maintain for 21 days and test negative (PCR) for release.
Maintain other mammalian pets under household isolation with regular vet checks to ensure no clinical signs are observed.
This risk assessments addresses:
- the overall likelihood that an animal will become infected with monkeypox virus through close direct and prolonged contact in a household with an infected human, and that this could lead to further human infections
- the assumption that the naïve animal has already had contact with a known human confirmed case and subsequently became infected would depend on the species of animal and the clinical severity of the human case or level of contamination in the environment
- the risk pathway for further transmission to humans, which is not only direct contact between a naïve human and the infected pet, but the potential for the infected pet to transmit to endemic wild, peridomestic or domestic animals, through direct contact or contact with a contaminated environment, and then to humans
- this risk assessment does not address human-to-human transmission of monkeypox virus
Step 1. Assessment of the probability of infection in the UK human population
This section of the assessment examines the likelihood of an infectious threat causing infection in the UK human population. Where a new agent is identified there may be insufficient information to carry out a risk assessment and this should be clearly documented. Please read in conjunction with the Probability Algorithm found at Annex A.
In this circumstance, we are assuming monkeypox virus infection is already present in a person owning a pet in the UK, and that the pet may become infected, giving rise to more human cases.
Transmission is likely to be via close contact with the infected animal (bites, scratches, respiratory droplets) or contaminated fomites in the household setting. We are considering the susceptibility of the pet species involved, and not the likelihood of the pet becoming infected from the infected human and/or contaminated environment.
Is this a recognised human disease?
Outcome: yes.
Quality of evidence: good – humans; satisfactory – rodents; poor – other species.
Monkeypox (MPX) is a rare zoonosis that can cause human illness clinically indistinguishable from smallpox (1). It is caused by the monkeypox virus (MPXV), which is an orthopoxvirus that is genetically distinct from other members of the Poxviridae family, including the variola, vaccinia, ectromelia, camelpox, and cowpox viruses. It was first identified as the cause of a pox-like illness in captive monkeys at the State Serum Institute in Copenhagen in 1958 (2).
MPX is regarded as the most important orthopoxvirus infection in human beings since the eradication of smallpox (3) By contrast with variola virus, however, MPXV has a wide range of hosts (4), which has allowed it to maintain a reservoir in wild animals while sporadically causing human disease (13). It therefore cannot be eradicated by human vaccination alone, although it may be possible to be controlled by targeted vaccination of humans (using a smallpox vaccine, which is approved for use against MPX) and culling animal reservoirs in certain circumstances.
The first human case of MPX was recorded in 1970 in the Democratic Republic of the Congo (DRC) (5), and since then the infection has been reported in a number of central and western African countries, where it is considered endemic. Most human cases are reported from the DRC and Nigeria, with clusters of MPX cases occasionally being reported in Central African Republic (CAR).
Sporadic cases have been reported in Cameroon, Gabon, Côte d’Ivoire, Liberia, the Republic of the Congo, Sierra Leone, and South Sudan (6 to 11).
In 2022, outbreaks of MPX have been reported in Cameroon, CAR, the DRC and Nigeria (12), although the true number of cases may be under-reported given limited surveillance and diagnostic capacities in these countries.
There are 2 clades of MPXV:
- Clade I (formerly Congo Basin clade) found predominantly in the DRC and CAR
- Clade II (formerly West African clade, historically found in Sierra Leone, Nigeria, Liberia, Ivory Coast, and Gambia)
The case fatality rate (CFR) can vary between 1% and 10%, but it is clade dependent with Clade II causing lower mortality (13) To date, Cameroon is the only country where both clades have been detected (13). Clade II consists of the subclades Clade IIa and IIb, with the latter subclade referring mainly to the group of variants circulating in the 2022 global outbreak (51).
In 2003, a large outbreak of human cases of MPX was detected in the USA, which was associated with the importation of pet rodents from West Africa. Laboratory testing by the US Centers for Disease Control (CDC) and Prevention showed that 2 African giant pouched rats, 9 dormice and 3 rope squirrels were infected with MPXV.
After importation into the USA, some of the infected animals were housed near prairie dogs at the facilities of an Illinois animal vendor. These prairie dogs were sold as pets before they developed signs of infection. Consequently, there were 47 confirmed and probable human cases (no deaths), and no human-to-human transmission was reported (14).
Is the disease endemic in the UK?
Outcome: no.
Quality of evidence: good.
MPX is not an endemic disease in the UK. Prior to 2022, 7 cases of MPX had been reported (all Clade II) in the UK, all of which were, or were linked to, travel associated cases. In 2018, 2 cases of MPX were identified in the UK in individuals who had travelled from Nigeria (15). The cases were epidemiologically unconnected.
A third case was diagnosed with MPX in 2018, following contact with contaminated bed linen. The case was a healthcare worker involved in the care of one of the previous cases (16). In 2019, an individual in England was confirmed to have MPX after recently travelling from Nigeria.
In May 2021, a case of MPX was identified in Wales, also with a travel history from Nigeria. In this incident, 2 family members were subsequently identified as having MPX and all 3 cases recovered (17).
On 7 May 2022, the UK Health Security Agency (UKHSA) announced a confirmed case of MPX in an individual with a travel history to Nigeria (18). The case developed a rash on 29 April and arrived in the UK on 4 May, having departed Nigeria on 3 May. Extensive contact tracing was undertaken to identify and follow-up exposed contacts of the case in healthcare settings, the community and the international flight.
On 14 May, 2 MPX cases were identified in London who were not linked to the case reported on the 7 May (18).
Four new cases were identified on the 16 May; 3 in London and 1 case in the North East of England (18). None of these cases had known links to those reported on the 7 and 14 May and there was no link to travel to a country where MPX is endemic.
Genomic sequencing confirmed the cases were infected with the Clade II of the virus (18).
As of 14 August 2022, a total of 35,275 laboratory confirmed cases of monkeypox, including 12 deaths, had been reported across 92 countries/territories/areas. Countries reporting the greatest number of cases included the USA (10,725 cases), Spain (5,719 cases), Germany (3,102 cases), UK (3,037 cases), France (2,673 cases), Brazil (2,584 cases), Canada (1,059 cases), Netherlands (1,025 cases), Portugal (770 cases) and Peru (653 cases) (19).
Are there routes of introduction into the UK?
Outcome: yes.
Quality of evidence: satisfactory.
The risk of new introductions of MPX to the UK depends on the extent of the circulation of the virus in other countries. In the past, individuals with a travel history to Nigeria and the DRC were considered as those of highest risk of importation of MPX.
Given the geographical expansion of non-endemic countries now reporting confirmed cases of MPX (notable Clade IIb) and community transmission in some of these countries – the extent of which is under investigation – then new travel associated cases in the UK cannot be excluded.
The risk to the UK public would come from a human case of MPX being imported into the UK, which is dependent on the number of travellers expected from affected countries and direct, rapid travel routes, or the import of an infected animal reservoir.
The most recent cases are not the first to be reported in the UK, but are unusual with respect to the wider human-to-human transmission.
The movement of primates or rodents for commercial reasons or as pets into Great Britain is regulated by the Rabies Import Order (The Rabies (Importation of Dogs, Cats and Other Mammals) Order 1974), and the Rabies (Importation of Dogs, Cats and Other Mammals) Order (Northern Ireland) 1977, which would require all animals to undergo 4 months quarantine (3 months in Scotland) in a rabies-approved quarantine kennel after entry, unless prepared for pet travel or a derogation is applied (such as for approved establishments like zoos).
Commercial movements from Europe and their large exotic pet markets may be undertaken under licence and do not require a health certificate. Quarantine establishment kennels are not necessarily rodent proof, but are designed to prevent the escape or deliberate release of animals and to manage transmission of rabies between those animals present.
Are effective control measures in place to mitigate against these routes of introduction?
Outcome: yes – humans; no – rodents and non-rodent mammalian animals.
Quality of evidence: satisfactory.
Human-to-human transmission may occur through contact with clothing or linens (such as bedding or towels) used by an infected person, direct contact with MPX skin lesions or scabs, or through respiratory droplets when an infected person with a MPX rash coughs or sneezes (13).
Thus, preventing direct contact is the most appropriate control measure. Human cases can be treated and ring vaccination (using smallpox vaccine) of contacts can be applied. After infection, and once the scabs have dried up, the patient is no longer infectious and there is a strong immune response.
Effective decontamination of the environment frequented by a person with MPX is also necessary (20). Whilst the virus (enveloped DNA virus) is highly resistant in the environment to temperature, it can be destroyed with detergents.
If infection is present in small mammals which are imported as part of the exotic pet trade from the European Union (EU), there are no effective control measures to prevent imports. No border checks are in place for the movement of such animals and there is no health certificate required. Commercial documents are not routinely examined for such imports.
There are no harmonised health certificates for the commercial import of rodents into the EU from third countries, and MPX is not notifiable under the EU Animal Health Law Commission Implementing Regulation EU/2018/1882.
Once imported into the EU, they would not be subject to quarantine on arrival in the UK, unless they are declared as originating in a third country, because derogations are routinely given for EU-origin animals. Exotic pet trade fairs are commonplace in the EU.
Therefore, susceptible mammalian pets that were in direct close contact with an infected human should be isolated for a period equivalent to the human maximum incubation period of 21 days. Veterinary management of animal patients involves symptomatic treatment and supportive care, as required.
Do environmental conditions in the UK support the natural reservoirs or vectors of disease?
Outcome: yes.
Quality of evidence: satisfactory.
Various animal species have been identified as susceptible to MPXV. Rodents are considered natural reservoirs of infection, including rope squirrels, tree squirrels, Gambian pouched rats, dormice, non-human primates (21) and other species (22).
Uncertainty remains concerning the natural history of MPXV and further studies are needed to identify the exact reservoir(s) and how virus circulation is maintained in nature. However, there is laboratory evidence of susceptibility for the red squirrel, Sciurus vulgaris (22) and for certain wild derived strains of Mus musculus, the house mouse.
Although there is no evidence of susceptibility of common endemic wildlife species in the UK to MPXV, rodents (including voles, dormice, rats, mice, and squirrels) as well as lagomorphs (rabbits and hares) and hedgehogs could all be considered potential reservoirs, based on field and laboratory observations (22).
Investigating small animals such as rabbits, ground squirrels, prairie dogs, cotton rates, white rats, golden hamsters and guinea pigs as animal models for MPXV demonstrated varying levels of susceptibility depending on route of inoculation and age of the animal (23).
Lack of clinical signs in infected adult white rats and white rabbits supports the theory that the clinical outcome will depend on the route of inoculation or age and immune status of the animals (23). This, coupled with potential for viral shedding and limited data on pathogenesis, provides low confidence in diagnostic predictive values using different sampling matrices or in the incubation period.
In 1979, a large-scale survey of animals (representing at least 43 species) in the DRC detected evidence of positive serology for Orthopoxviruses among non-human primates, as well as evidence of at least one species of terrestrial rodent, mainly squirrels, exhibiting presumed MPXV-specific reactivity (24). This was consistent with findings that ~12% of persons presumed to have been infected by contact with animals had recent contact with squirrels (25).
None of the domestic animals tested – 120 sheep and goats, 67 cats – exhibited serological evidence of Orthopoxvirus infection (26). In 1985, the isolation of MPXV from a captured, symptomatic squirrel (Funiscirurs anerythrus) was made (24).
The only other instance of virus isolation from a wild animal was in 2012 from a juvenile sooty mangaby (Cercocebus atys) from Côte d’Ivoire (27).
The range of taxa capable of supporting infection with MPXV is wide, though several common peridomestic rodents have been ruled out. Adult white rabbits and white rats (genus Rattus) have been observed to be refractory to infection with MPXV, but not newborns (reviewed in (5)).
Nearly all sub-species of the common house mouse, Mus musculus, are resistant to challenge with MPXV when adult animals with functional immune systems are used (28).
One exception to this is the castaneous (CAST) subspecies of the house mouse, Mus musculus castaneus, due to intrinsic low levels of IFN-γ and TNF-α responses and overall fewer NK cells and CD4+ and CD8+ T cells (29).
Outbreaks among captive animals on display or kept as pets reveal further evidence for the involvement of animals. Most recently, monkeypox infection was reported in a dog in France (52) and a dog in Brazil (53), likely as a result of human to animal transmission following direct contact while the humans were symptomatic. The dogs showed mucocutaneous lesions and tested positive on PCR. These are the first documented cases of human to animal transmission of monkeypox virus.
Historically, 2 New World giant anteaters (Myrmecophaga tridactyla) were infected with MPX at the Rotterdam zoo outbreak in 1964, during which individuals from 7 different species of non-human primates became ill and in some cases died (30).
While anteaters are not considered to play a role in the natural lifecycle of MPXV, it is possible that transmission hosts need not be natural, maintenance hosts of the virus. Captive animals seem particularly vulnerable to the epizootic spread of MPXV, whether due to crowding, species mixing, or physiological stress, a fact underscored by 2 outbreaks at primate sanctuaries in Cameroon (31).
Under experimental conditions, susceptibility of rabbits to MPXV has been shown (32) and infection of an African hedgehog was confirmed in the 2003 USA outbreak (33). The susceptibility of wild or pet/captive Mustelidae to MPXV is unknown.
In 2003, in the USA, MPXV was introduced to several mid-western states through a consignment of African rodents (origin Ghana) destined for the pet trade (34). Local surveillance at the site of animal carcass disposal did not detect evidence of the virus in feral/wild rodent populations.
Three genera of African rodent, Graphiurus, Cricetomys and Funisciurus (African dormice, giant pouched rat, rope squirrel, respectively) were implicated as vehicles of virus introduction during the initial importation event (32), (35).
Studies performed subsequently to assess the competence of each species to serve as natural reservoirs of the virus demonstrated that, in general, though none showed ‘tolerance’ (for example, virus amplification and shedding in the absence of evident disease), each was capable of being infected and of shedding viable virus for extended periods of time through varied routes (36 to 40).
Pox viruses, including MPXV, are very stable in the environment and therefore exposure of wild or pet rodents to contaminated household rubbish cannot be excluded. Wildlife involvement was ruled out in the cases in the USA in 2003, where wildlife surveillance was conducted, and surveillance around outbreaks in Nigeria have not found infected wild trapped rodents, but more research is needed in this area. An effective isolation period should therefore take into account the date from when the household has been decontaminated or the pet removed from the environment.
Will there be human exposure?
Outcome: no – general population; yes – for individuals interacting with infected pets (notably rodents) and their contaminated environments.
Quality of evidence: good.
MPXV enters the body through broken skin (even if the wound is not visible), respiratory tract, or the mucous membranes (eyes, nose, or mouth).
Human-to-human transmission is thought to occur either through large respiratory droplets (these respiratory droplets generally cannot travel more than a few feet, and so prolonged face-to-face contact is required), direct contact with body fluids or lesion material, and indirect contact with clothing or linens (such as bedding or towels) used by an infected person.
Animal-to-human transmission may occur when a person comes into close contact with the saliva, blood or other bodily fluids of an infected animal. If clinical signs, such as pustules and skin lesions are present, the process of desquamation can lead to environmental contamination.
Exposures may include being bitten or scratched by an infected animal, preparation of infected wild animal carcasses for consumption, or contact with a contaminated environment frequented by an infected animal (such as contaminated animal bedding, feeding bowls, water bottles).
Pet owners of fancy rats and other domestically kept rodents are usually in close contact with their pets, and their environments (for example housing, bedding, feeding materials). Based on transmission pathways of Hanta- (41) and Borna-viruses (42), there are known risk pathways for transmission, including cleaning cages.
The MPXV is an ACDP Category 3 pathogen, meaning it can cause severe human disease and may be a serious hazard to those handling and processing samples (for example healthcare workers, laboratory staff) if appropriate personal protective equipment is not worn.
Are humans highly susceptible?
Outcome: yes.
Quality of evidence: good.
MPX is usually a self-limited disease with symptoms lasting from 2 to 4 weeks. The illness is often mild and most of those infected will recover without treatment. Cases of severe disease can occur more commonly among children and are related to the extent of virus exposure, patient health status and nature of complications. Underlying immune deficiencies may lead to worse outcomes.
Smallpox vaccine, cidofovir, and tecovirimat can be used to control outbreaks of MPX (43). Vaccination against smallpox can be used for both pre- and post-exposure and is up to 85% effective in preventing MPX disease (13).
People vaccinated against smallpox in childhood may experience a milder disease. People younger than 40 to 50 years of age (depending on the country) may be more susceptible to MPX due to cessation of smallpox vaccination campaigns globally after eradication of the disease.
The extent to which asymptomatic infection may occur is unknown.
Is this disease highly infectious in humans?
Outcome: yes/no.
Quality of evidence: satisfactory.
Historically, cases of MPX in humans have been considered rare and zoonotic. In recent years, there has been increased reports of cases from endemic countries. Most outbreaks in the past have been short-lived and self-limiting, with only limited human-to-human transmission.
People vaccinated against smallpox in childhood may experience a milder disease. Immunity in those who were vaccinated against smallpox would confer cross protection, but this population is declining in most countries.
Generally, pox viruses do not have high reproductive rates and thus may not be considered highly infectious, however the reproductive rate for the outbreaks in Europe is not known and therefore we cannot apply this to a human-to-animal infection. Close contact with an infected human or animal is required for transmission to take place.
Infectiousness may be considered higher for individuals partaking in activities which would result in close skin contact or exposure to infectious air droplets and/or bodily fluids or material from desquamation (43).
Outcome of probability assessment
The probability of human infection with MPXV in the general UK population is considered very low.
For individuals interacting with infected pets (notably rodents) and their contaminated environments, the probability would be considered moderate to high.
Step 2: Assessment of the impact on human health
The scale of harm caused by the infectious threat in terms of morbidity and mortality: this depends on spread, severity, availability of interventions and context.
Please read in conjunction with the impact algorithm found at Annex B.
Is there human-to-human spread of this pathogen?
Outcome: yes.
Quality of evidence: good.
Human-to-human transmission of MPX may occur through contact with clothing or linens (such as bedding or towels) used by an infected person, direct contact with MPX skin lesions or scabs and/or through coughing or sneezing of an individual with a MPX rash.
Human-to-human transmission can also occur through large respiratory droplets, which generally cannot travel more than a few feet, and so prolonged face-to-face contact with an infected person is required. Individuals that may have direct skin contact with an infected individual may have a greater exposure risk to MPX infection.
Is the UK human population susceptible?
Outcome: yes.
Quality of evidence: good.
See above evidence.
Does it cause severe disease in humans?
Outcome: yes/no.
Quality of evidence: satisfactory.
MPX is usually a self-limited disease with symptoms lasting from 2 to 4 weeks. The illness is often mild and most of those infected will recover without treatment. Disease usually begins with fever, myalgia, fatigue and a headache (44).
Within 1 to 5 days after the appearance of fever, a rash develops, often beginning on the face then spreading to other parts of the body. The rash progresses through different stages before finally forming a scab which later falls off.
The onset of rash is typically considered the start of the infectious period (45), and an individual is contagious until all the scabs have fallen off and there is intact skin underneath. The scabs may also contain infectious virus material.
Severe cases occur more commonly among children (13) and are related to the extent of virus exposure, patient health status and the nature of complications. In endemic countries, complications include secondary infections, bronchopneumonia, sepsis, encephalitis, and infection of the cornea impeding vision (46).
Underlying immune deficiencies may also lead to worse outcomes. Disease severity is also dependent on the MPXV clade an individual is infected with.
Historically, the less common Clade I has caused more severe disease (CFR up to 10%) and may be more transmissible compared to Clade II (CFR approximately 1%) (47, 48).
Is it highly infectious to humans?
Outcome: yes/no.
Quality of evidence: satisfactory.
See above evidence.
Are effective interventions (preventative or therapeutic) available?
Outcome: yes.
Quality of evidence: good.
There are no vaccines or treatments that have been developed specifically for MPX. Clinical care for MPX is mainly supportive. Patients should be offered fluids and food to maintain adequate nutritional status and secondary bacterial infections should be prevented and treated (13).
An antiviral agent known as tecovirimat, that was developed for smallpox, was licensed by the European Medical Association (EMA) for MPX in 2022 based on data in animal and human studies. Smallpox vaccine, cidofovir, and tecovirimat can be used to control outbreaks of MPX (43).
Vaccination against smallpox can be used for both pre- and post-exposure, and is up to 85% effective in preventing MPX disease (13).
Appropriate respiratory isolation of suspected and confirmed MPX cases is essential for preventing transmission by respiratory and contact routes. Scabs are also infectious and care must be taken to avoid infection through handling contaminated clothing or linen (such as bedding or towels) that has been used by an infected person.
Individuals cleaning or decontaminating rooms that a patient with MPX has spent significant time in should wear appropriate personal protective equipment to avoid direct contact with contaminated material during the decontamination process (20).
Isolation of pets to prevent further contacts with uninfected people should be applied to non-rodent pet animals as a precaution, and provided the welfare of the animal is not compromised. However, where there are rodent pets present, the evidence base is stronger that they can contribute to MPXV spread amongst rodents and to people in direct contact (43).
The rodent pets that were exposed to the virus in a contaminated household should not be handled directly and should be prevented from contact with any wild or peridomestic rodents.
Would a significant number of people be affected?
Outcome: no.
Quality of evidence: good.
Within the UK, well established and robust public health interventions are implemented in response to cases of MPX infection. This involves the isolation and treatment (if required) of suspected and confirmed MPX cases, and where appropriate extensive contact tracing designed at preventing further transmission.
Ring vaccination, using the modified vaccinia Ankara (MVA-BN) (Imvanex) Smallpox vaccine, has also been used in the UK in response to MPX incidents (49).
There is no requirement for pet rodents to be registered in the UK. Predicted numbers of pet rodents in the UK is based mainly on sales data for pet food and is estimated at around 2 million households (as opposed to 12 million each for cats and dogs) (50). There is no data for imports of ‘exotic pets’.
As most rodents are kept in cages within the household, and may be handled frequently, the level of exposure to owners is likely to be high, but less so for visitors or household members who are not comfortable with handling small mammals. Therefore, the level of exposure at the population level is considered low.
Contact with peridomestic rodents (house mice predominantly, but rats are commonly found in urban settings) cannot be excluded for any household with pet rodents. Therefore, there would be a potential exposure route for peridomestic rodents, but these animals are even less likely to have close direct contact with a human, nevertheless, the transmission pathways for wild animals to humans have not been fully elucidated and contact with contaminated surfaces cannot be ruled out.
Outcome of impact assessment
The impact of MPX on human health in the UK is considered very low to low.
Annex A. Assessment of the probability of infection in the UK population algorithm
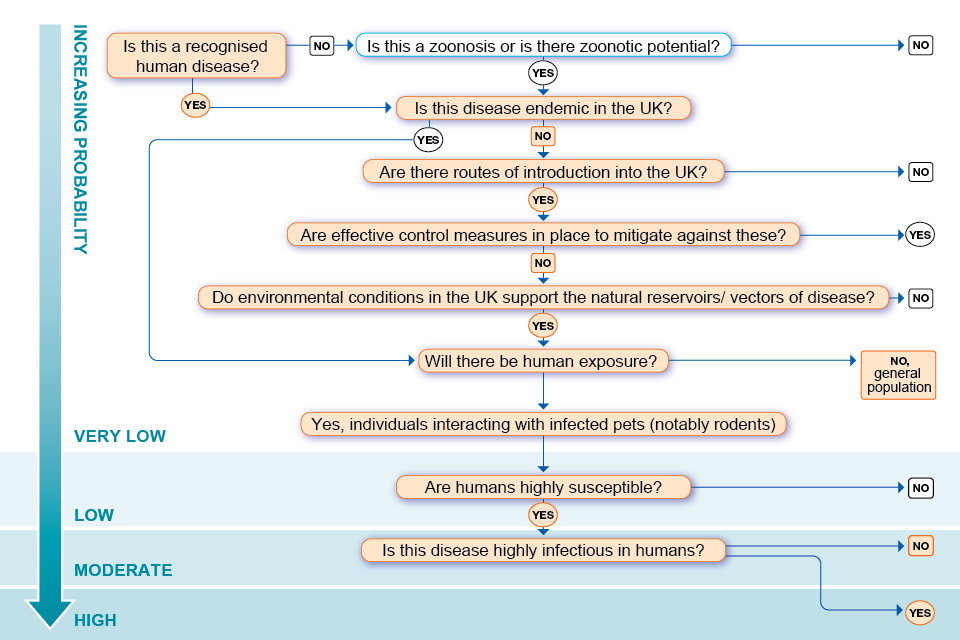
Accessible text version of Annex A
Outcomes are specified by a ☑ (tick) beside the appropriate answer.
Question 1: Is this a recognised human disease?
Yes: go to question 3 ☑
No: go to question 4
Question 2: Is this a zoonosis or is there zoonotic potential
Yes: go to question 3
No: probability of infection in UK population is very low
Question 3: Is this disease endemic in the UK?
Yes: go to question 7
No: go to question 4 ☑
Question 4: Are there routes of introduction into the UK?
Yes: go to question 5 ☑
No: probability of infection in UK population is very low
Question 5: Are effective control measures in place to mitigate against these?
Yes: probability of infection in UK population is very low
No: go to question 6 ☑
Question 6: Do environmental conditions in the UK support the natural reservoirs/vectors of disease?
Yes: go to question 7 ☑
No: probability of infection in UK population is very low
Question 7: Will there be human exposure
Yes: Individuals interacting with infected pets (notably rodents): Go to question 8 ☑
No: probability of infection in general UK population is very low ☑
Question 8: Are humans highly susceptible?
Yes: go to question 9 ☑
No: probability of infection in UK population is low
Question 9: Is this disease highly infectious in humans?
Yes: probability of infection in individuals interacting with infected pets (notably rodents) is high. ☑
No: probability of infection in individuals interacting with infected pets (notably rodents) is moderate. ☑
Annex B. Assessment of the impact on human health algorithm
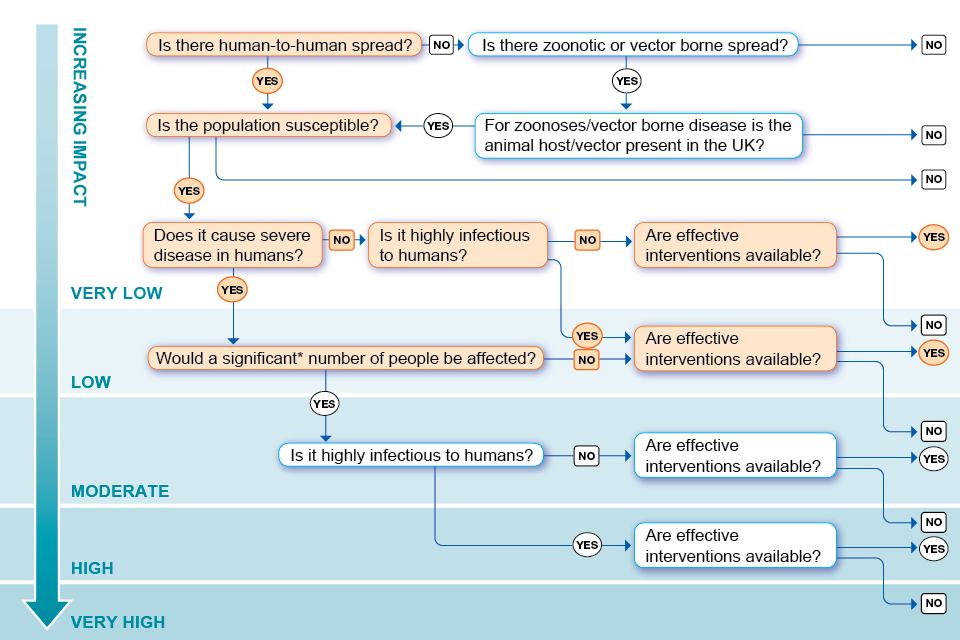
*This question has been added to differentiate between those infections causing severe disease in a handful of people and those causing severe disease in larger numbers of people. ‘Significant’ is not quantified in the algorithm but has been left open for discussion and definition within the context of the risk being assessed.
Accessible text version of Annex B
Outcomes are specified by a ☑ (tick) beside the appropriate answer.
Question 1: Is there human-to-human spread?
Yes: go to question 4 ☑
No: go to question 2
Question 2: Is there zoonotic or vector borne spread?
Yes: go to question 3
No: impact on human health in the UK is very low
Question 3: Is the animal host or vector present in the UK?
Yes (animal host): go to question 4
No (vector): impact on human health in the UK is very low
Question 4: Is the population susceptible?
Yes: go to question 5 ☑
No: impact on human health in the UK is very low
Question 5: Does it cause severe human disease?
Yes (immunocompromised individuals): go to question 8 ☑
No: go to question 6 ☑
Question 6: Is it highly infectious to humans?
Yes: go to question 9 ☑
No: go to question 7 ☑
Question 7: Are effective interventions available?
Yes: impact on human health in the UK is very low ☑
No: impact on human health in the UK is low
Question 8: Would a significant number of people be affected?
Yes: go to question 10
No: go to question 9 ☑
Question 9: Are effective interventions available?
Yes: impact on human health in the UK is low ☑
No: impact on human health in the UK is moderate
Question 10: is it highly infectious to humans?
Yes: go to question 12
No: go to question 11
Question 11: Are effective interventions available?
Yes: impact on human health in the UK is moderate
No: impact on human health in the UK is high
Question 12: Are effective interventions available?
Yes: impact on human health in the UK is high
No: impact on human health in the UK is very high
References
1. Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. The Lancet Infectious Diseases. 2004;4(1):15-25.
2. Magnus Pv, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathologica Microbiologica Scandinavica. 1959;46(2):156-76.
3. World Health Organization (WHO). The global eradication of smallpox: final report of the Global Commission for the Certification of Smallpox Eradication, Geneva, December 1979: WHO; 1980.
4. Gispen R. Relevance of some poxvirus infections in monkeys to smallpox eradication. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1975;69(3):299-302.
5. Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future virology. 2013;8(2):129-57.
6. WHO. Weekly Bulletin on Outbreaks and other Emergencies: Week 3: 13 to 19 January 2020.
7. WHO. Weekly Epidemiological Record, 2018, vol. 93, 11 [full issue]. Weekly Epidemiological Record; Relevé épidémiologique hebdomadaire. 2018;93(11):117-32.
8. Larway LZ, Amo-Addae M, Bulage L, Adewuyi P, Shannon F, Wilson W, and others. An Outbreak of Monkeypox in Doedain District, Rivercess County, Liberia, June, 2017. Journal of Interventional Epidemiology and Public Health. 2021;4(8).
9. Doshi RH, Guagliardo SAJ, Doty JB, Babeaux AD, Matheny A, Burgado J, and others. Epidemiologic and ecologic investigations of monkeypox, Likouala Department, Republic of the Congo, 2017. Emerging Infectious Diseases. 2019;25(2):273.
10. Reynolds MG, Wauquier N, Li Y, Satheshkumar PS, Kanneh LD, Monroe B, and others. Human monkeypox in Sierra Leone after 44-year absence of reported cases. Emerging Infectious Diseases. 2019;25(5):1023.
11. Formenty P, Muntasir MO, Damon I, Chowdhary V, Opoka ML, Monimart C, and others. Human monkeypox outbreak caused by novel virus belonging to Congo Basin clade, Sudan, 2005. Emerging infectious diseases. 2010;16(10):1539.
12. WHO. Weekly Bulletin on Outbreaks and other Emergencies: Week 17: 18 to 24 April 2022.
13. WHO. Monkeypox fact sheet. 2022.
14. Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, and others. Clinical manifestations of human monkeypox influenced by route of infection. The Journal of Infectious Diseases. 2006;194(6):773-80.
15. Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, and others. Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance. 2018;23(38):1800509.
16. Vaughan A, Aarons E, Astbury J, Brooks T, Chand M, Flegg P, and others. Human-to-human transmission of monkeypox virus, UK, October 2018. Emerging Infectious Diseases. 2020;26(4):782.
17. Public Health England (PHE). Monkeypox case confirmed in England. 2019.
18. UKHSA. Monkeypox cases confirmed in England – latest updates. 2022.
19. WHO. 2022 Monkeypox Outbreak: Global Trends 2022.
20. PHE. Monkeypox: Guidance for environmental cleaning and decontamination. 2018.
21. Silva NIO, de Oliveira JS, Kroon EG, Trindade GdS, Drumond BP. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses. 2020;13(1):43.
22. Reynolds MG, Doty JB, McCollum AM, Olson VA, Nakazawa Y. Monkeypox re-emergence in Africa: a call to expand the concept and practice of One Health. Expert review of anti-infective therapy. 2019;17(2):129-39.
23. Hutson CL, Damon IK. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2(12):2763-76.
24. Khodakevich L, Jezek Z, Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Isolation of monkeypox virus from wild squirrel infected in nature. 1986 (Jan 11):98-9.
25. Arita I, Jezek Z, Khodakevich L, Ruti K. Human monkeypox: a newly emerged orthopoxvirus zoonosis in the tropical rain forests of Africa. The American Journal of Tropical Medicine and Hygiene. 1985;34(4):781-9.
26. Khodakevich L, Szczeniowski M, Jezek Z, Marennikova S, Nakano J, Meier F. Monkeypox virus in relation to the ecological features surrounding human settlements in Bumba zone, Zaire. Tropical and geographical medicine. 1987;39(1):56-63.
27. Radonic A, Metzger S, Dabrowski PW, Couacy-Hymann E, Schuenadel L, Kurth A, and others. Fatal monkeypox in wild-living sooty mangabey, Côte d’Ivoire, 2012. Emerging Infectious Diseases. 2014;20(6):1009.
28. Americo JL, Moss B, Earl PL. Identification of wild-derived inbred mouse strains highly susceptible to monkeypox virus infection for use as small animal models. Journal of Virology. 2010;84(16):8172-80.
29. Americo JL, Sood CL, Cotter CA, Vogel JL, Kristie TM, Moss B, and others. Susceptibility of the wild-derived inbred CAST/Ei mouse to infection by orthopoxviruses analyzed by live bioluminescence imaging. Virology. 2014;449:120-32.
30. Gispen R, Verlinde J, Zwart P. Histopathological and virological studies on monkey-pox. Archiv fur die gesamte Virusforschung. 1967;21(2):205-16.
31. Durski KN, McCollum AM, Nakazawa Y, Petersen BW, Reynolds MG, Briand S, and others. Emergence of monkeypox─west and central Africa, 1970 to 2017. Morbidity and mortality weekly report. 2018;67(10):306.
32. Marennikova S, Seluhina E. Susceptibility of some rodent species to monkeypox virus, and course of the infection. Bulletin of the World Health Organization. 1976;53(1):13.
33. Bernard SM, Anderson SA. Qualitative assessment of risk for monkeypox associated with domestic trade in certain animal species, United States. Emerging Infectious Diseases. 2006;12(12):1827.
34. Hutson CL, Lee KN, Abel J, Carroll DS, Montgomery JM, Olson VA, and others. Monkeypox zoonotic associations: insights from laboratory evaluation of animals associated with the multi-state US outbreak. The American journal of tropical medicine and hygiene. 2007;76(4):757-68.
35. CDC. Multistate outbreak of monkeypox – Illinois, Indiana, and Wisconsin, 2003. MMWR Morbidity and mortality weekly report. 2003;52(23):537-40.
36. Earl PL, Americo JL, Cotter CA, Moss B. Comparative live bioluminescence imaging of monkeypox virus dissemination in a wild-derived inbred mouse (Mus musculus castaneus) and outbred African dormouse (Graphiurus kelleni). Virology. 2015;475:150-8.
37. Falendysz EA, Lopera JG, Lorenzsonn F, Salzer JS, Hutson CL, Doty J, and others. Further assessment of monkeypox virus infection in Gambian pouched rats (Cricetomys gambianus) using in vivo bioluminescent imaging. PLoS Neglected Tropical Diseases. 2015;9(10):e0004130.
38. Falendysz EA, Lopera JG, Doty JB, Nakazawa Y, Crill C, Lorenzsonn F, and others. Characterization of Monkeypox virus infection in African rope squirrels (Funisciurus sp.). PLoS neglected tropical diseases. 2017;11(8):e0005809.
39. Hutson CL, Nakazawa YJ, Self J, Olson VA, Regnery RL, Braden Z, and others. Laboratory investigations of African pouched rats (Cricetomys gambianus) as a potential reservoir host species for monkeypox virus. PLoS neglected tropical diseases. 2015;9(10):e0004013.
40. Schultz DA, Sagartz JE, Huso DL, Buller RML. Experimental infection of an African dormouse (Graphiurus kelleni) with monkeypox virus. Virology. 2009;383(1):86-92.
41. PHE. HAIRS risk assessment: hantavirus. 2016.
42. PHE. HAIRS risk assessment: squirrel Bornavirus. 2019.
43. Centers for Disease Control and Prevention (CDC). Monkeypox: treatment. 2021.
44. Yinka-Ogunleye A, Aruna O, Ogoina D, Aworabhi N, Eteng W, Badaru S, and others. Reemergence of human monkeypox in Nigeria, 2017. Emerging infectious diseases. 2018;24(6):1149.
45. Brown K, Leggat PA. Human monkeypox: current state of knowledge and implications for the future. Tropical medicine and infectious disease. 2016;1(1):8.
46. Hughes C, McCollum A, Pukuta E, Karhemere S, Nguete B, Lushima RS, and others. Ocular complications associated with acute monkeypox virus infection, DRC. International Journal of Infectious Diseases. 2014;21:276-7.
47. Nakazawa Y, Mauldin MR, Emerson GL, Reynolds MG, Lash RR, Gao J, and others. A phylogeographic investigation of African monkeypox. Viruses. 2015;7(4):2168-84.
48. Sadeuh-Mba SA, Yonga MG, Els M, Batejat C, Eyangoh S, Caro V, and others. Monkeypox virus phylogenetic similarities between a human case detected in Cameroon in 2018 and the 2017-2018 outbreak in Nigeria. Infection, Genetics and Evolution. 2019;69:8-11.
49. UKHSA. Recommendations for the use of pre and post exposure vaccination during a monkeypox incident. 2022.
50. Pet Food Manufacturers Association (PFMA). Pet Population 2021.
51. World Health Organisation (WHO). Monkeypox: experts give virus variants new names. 2022.
52. Seang S, Burrel S, Todesco E, Leducq V, Monsel G, Le Pluart D, and others. Evidence of human-to-dog transmission of monkeypox virus. The Lancet. 2022.
53. Minas Gerais State Health Department. Detection of monkeypox in an animal in Minas Gerais. 2022
