Abstract
The end-Permian mass extinction (EPME) was the most severe extinction event in the past 540 million years, and the Siberian Traps large igneous province (STLIP) is widely hypothesized to have been the primary trigger for the environmental catastrophe. The killing mechanisms depend critically on the nature of volatiles ejected during STLIP eruptions, initiating about 300 kyr before the extinction event, because the atmosphere is the primary interface between magmatism and extinction. Here we report Ni isotopes for Permian-Triassic sedimentary rocks from Arctic Canada. The δ60Ni data range from −1.09‰ to 0.35‰, and exhibit the lightest δ60Ni compositions ever reported for sedimentary rocks. Our results provide strong evidence for global dispersion and loading of Ni-rich aerosol particles into the Panthalassic Ocean. Our data demonstrate that environmental degradation had begun well before the extinction event and provide a link between global dispersion of Ni-rich aerosols, ocean chemistry changes, and the EPME.
Similar content being viewed by others
Introduction
The end-Permian mass extinction (EPME) about 252 million years ago (Ma) was the most severe biotic crisis in the Phanerozoic, eliminating more than 90% of marine and 75% of terrestrial species1. The Siberian Traps large igneous province (STLIP), the largest known continental flood basalt province, is widely hypothesised to have been the primary trigger for the catastrophic environmental deterioration driving the EPME2,3,4,5,6. Potential kill mechanisms triggered by emplacement of the Siberian Traps magmas include global warming, ultraviolet radiation exposure, hypercapnia, ocean acidification and anoxia, and toxic metal release4,5,6,7,8,9,10,11,12,13,14. All of these potential mechanisms are critically dependent on the exact nature and timing of volatiles ejected during eruption because the atmosphere is the primary interface between STLIP magmatism and extinction. The initial eruption of the STLIP was about 300 ± 126 kyr prior to the onset of the EPME in marine realms3, during which the emplacement of the enormous Noril’sk nickel sulphide ore deposits in the Tunguska Basin may have released voluminous nickel-rich volcanic gas and aerosols into the atmosphere15. A spike in Ni abundance at the extinction level in the Meishan section, South China, is hypothesised to have triggered an explosive expansion of methanogenic Archaea and exponential increase of the marine inorganic carbon reservoir, leading to catastrophic mass extinction16. However, the Ni enrichment at Meishan could have also resulted from diagenesis17, and therefore the origin of that Ni spike needs to be further evaluated16. In addition, little is known about the potential timing of Ni loading to the oceans and how voluminous release and dispersal of Ni-rich aerosols may have impacted ocean chemistry, climate change, and ultimately the EPME. Here, we report Ni isotopes (δ60Ni) and Ni abundances for the Permian-Triassic sedimentary rocks from the Buchanan Lake section in the Sverdrup Basin, Canadian High Arctic. We use Ni isotopic and elemental data to study the climate and environmental consequences of emissions of Ni-rich aerosols and present a new scenario that links atmospheric and ocean chemistry changes triggered by the STLIP to the EPME.
Results and discussion
Geological setting and sampling
The Sverdrup Basin was a Carboniferous to Paleogene depocenter that accumulated over 12 km of sediment from Carboniferous to Paleogene time18 (Fig. 1). From Late Carboniferous to Early Triassic time, the Sverdrup Basin was along the NW margin of Pangea at palaeolatitudes of 35–40°N (ref. 19) (Fig. 1). Until the EPME, the basin was characterised by a central deep basinal area of fine-grained clastic deposition surrounded by a shallow shelf dominated by biogenic carbonate that transitioned in the late Permian to chert formed by shallow water siliceous sponges19. After the EPME, the Sverdrup basin was dominated by clastic-dominated sedimentation18. In this study, we examined the distal deep-water Buchanan Lake section which preserves outstanding Boreal records of the EPME, followed by the biotic recovery in the Early Triassic5. The Buchanan Lake section consists mostly of black shale of the Late Permian Black Stripe Formation and overlying Early Triassic Blind Fiord Formation that preserves characteristic post-extinction fauna20 (Fig. 2).
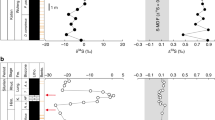
A Regional map. B Detailed map showing the location of the Buchanan Lake section. C Permian palaeogeographic map showing the location of the Sverdrup Basin relative to the Siberian Traps volcanic rocks (base map after C.R. Scotese [http://www.scotese.com/]). Wavy arrows indicate predominant westerly wind patterns.
During the last decade, the Buchanan Lake section has been extensively examined, and the carbon isotope chemostratigraphy, elemental compositions of the shale, and oceanic palaeo-redox changes have been well constrained5,11,19,20,21,22,23,24,25,26 (Fig. 2). The EPME in the Sverdrup Basin is marked by eradication of silica and carbonate producers along with the onset of a significant negative δ13Corg shift that has been correlated globally with the dated Global Stratotype Section and Point (GSSP) for the Permian-Triassic boundary at Meishan, China, at ~251.9 Ma (refs. 3,4,20,27,28) (Fig. 2). The palaeo-redox conditions during the deposition of the Late Permian Black Stripe Formation and Early Triassic Blind Fiord Formation evolved from an oxic water column with a strong redoxcline in the sediments to anoxic and then to sulphidic bottom water conditions (Fig. 2). The average total sulphur (TS) content for the samples from the oxic interval of the Black Stripe Formation (−86 m to −62 m) is 0.50 wt%, and the samples from the anoxic interval of the Black Stripe Formation (−62 to −2 m) have an average TS content of 0.89 wt%, except for one sample (C-445100) having TS content of 12.27 wt% (ref. 21). The sulphidic shales of the uppermost Black Stripe Formation are characterised by high TS contents with an average TS content of 2.09 wt% and small grains of framboidal pyrite5,21. The sulphidic shales of the lower Blind Fiord Formation have an average TS content of 1.15 wt% and contain pyrite layers of ~1 cm in thickness5,21 (Fig. 2).
Nickel isotopic and content data from the Buchanan Lake section
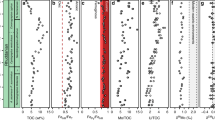
Nickel isotopic compositions (δ60Ni) are reported in delta notation relative to the Ni isotopic standard NIST SRM 986, in units of per mil (‰): [δ60/58Ni = (60Ni/58Nisample/60Ni/58NiSRM986 − 1) × 1000]. The δ60Ni compositions and Ni contents of the Buchanan Lake samples are presented in Fig. 2 (full analytical data are available as Supplementary Table 1). The δ60Ni and Ni contents show distinctive variations from pre-extinction to syn-extinction to post-extinction (Fig. 2).
Prior to the EPME, the δ60Ni values for the black shale (−86 to −62 m) deposited predominantly under oxic bottom water conditions show significantly light δ60Ni values ranging from −0.89‰ to −1.09‰, with Ni contents from 157.1 to 247.1 ppm (Fig. 2). The δ60Ni values for the black shale (−62 to −2 m) of the Black Stripe Formation deposited under anoxic bottom water conditions display a general increase from −0.99 to 0.32‰, although a minor variation up to 0.37‰ from −50 to −40 m is observed (Fig. 2). During the same interval, Ni contents vary between 117.5 and 247.1 ppm, although only a weak stratigraphic trend is exhibited, with decreasing Ni over time (Fig. 2).
The δ60Ni values for the black shale (−2 to 0 m) deposited under anoxic and sulphidic bottom water conditions of the Black Stripe Formation exhibit positive δ60Ni values from 0.07‰ to 0.34‰, and δ60Ni does not change significantly at the extinction horizon (Fig. 2). However, the Ni content drops sharply from 142.8 to 36.4 ppm at this level (Fig. 2). After the extinction, the δ60Ni values for the sulphidic black shale of the lower Blind Fiord Formation (0–19.4 m) vary between 0.00 and 0.34‰, and the Ni contents vary between 25.5 and 61.7 ppm (Fig. 2).
Nickel cycling and isotopic record in modern depositional systems
To evaluate possible interpretations of the Ni concentration and isotope profile in the Buchanan Lake section, we present a brief review of the Ni cycle and summarise the δ60Ni data that are closely relevant to our study (Fig. 3). Nickel is a bioessential trace metal, and, like phosphate and silica in the modern ocean, Ni exhibits a nutrient-type profile, with low concentrations in the photic zone due to biological uptake, and high concentrations at greater water depths due to the recycling of organic matter in the water column29. Nickel is also an essential component of important enzymes for the metabolism of methanogens and has been shown to have played a critical role in methane production and atmospheric oxygenation in the Archaean30,31,32. Like other stable isotope systems, the application of δ60Ni compositions to reconstruct Earth history depends on our understanding of the Ni cycling and δ60Ni variations in modern rivers, oceans, and sediments32,33,34. Dissolved Ni in modern oceans is mainly from: (1) weathering products of the continental crust transported by rivers to the oceans, (2) mineral dust and volcanogenic aerosols settling to the oceans from the atmosphere, and (3) hydrothermal vent fluids as a minor source34,35,36,37. The δ60Ni of dissolved Ni from riverine influx to the oceans has a heavy isotopic composition with an average δ60Ni value of +0.84‰ (ref. 36) (Fig. 3). The enrichment in heavy isotopes of riverine Ni relative to continental source rocks (δ60Ni mostly between −0.1‰ and +0.2‰)38 is likely caused by sorption of light Ni onto Fe oxides and authigenic clays during weathering37,39,40,41,42. Modern seawater has an average δ60Ni value of 1.44 ± 0.015‰, which is considerably heavier than the riverine inputs to the oceans36,43,44 (Fig. 3).
Rivers and the global ocean are from ref. 36, and Fe–Mn crusts are from refs. 35,45. Carbonates and OMZ sediments are from ref. 43. Magmatic sulphides are from refs. 38,48. Organic-rich black shale and mudstone are from ref. 47 and sulphidic Black Sea sediments are from ref. 33. The comparable light Ni isotopic compositions between the magmatic sulphides and our data are highlighted in orange.
The modern outputs of Ni from the ocean reservoir include sediments deposited under various redox conditions, as well as Fe–Mn crusts. Hydrogenic Fe–Mn crusts that potentially record the Ni isotopic composition of deep ocean water have a wide range of heavy δ60Ni values, from 0.25 to 2.5‰ with an average δ60Ni = 1.74 ± 0.59‰ (n = 126)35,45 (Fig. 3). The δ60Ni values for most of the sediments deposited either under oxic, suboxic, or anoxic conditions are similar to, or heavier than, seawater43. Therefore, at present, the budget of Ni isotopes at steady-state between inputs and outputs has an apparent imbalance35,36,37,43,45. However, δ60Ni values ranging from −0.2‰ to −0.8‰ were reported from the sediments that were deposited under oxygenated bottom water conditions at water depths of >3 km in the eastern Pacific46. These light δ60Ni values are interpreted to have resulted from diagenetic remobilisation, or mineralogical transformation of birnessite to todorokite, or possible scavenging of light Ni by Fe oxides or Fe-rich authigenic clays46.
We note that δ60Ni data from the oxygen minimum zone (OMZ) and the Black Sea sediments may be important in the interpretation of δ60Ni values in the rock record, especially in relation to ocean anoxic events that have occurred throughout Earth’s history. The δ60Ni values from Peru Margin OMZ sediments are between 1.11 and 1.21‰, with an average value of 1.16‰ (ref. 43) (Fig. 3). The Black Sea sulphidic sediments are characterised by lighter δ60Ni compositions ranging from 0.14 to 0.51‰ (Fig. 3), compared to the δ60Ni of 1.26‰ for dissolved Ni of the Danube River that enters the Black Sea33.
Regarding the geologic rock record, the only detailed δ60Ni studies so far are those of the organic-rich black shale sequence from the Jurassic Sinemurian-Pliensbachian GSSP at Robin Hood’s Bay, UK, and the Devonian–Mississippian Exshaw Formation in the West Canada Sedimentary Basin47 (Fig. 3). These black shales show δ60Ni values varying widely from 0.2 to 2.5‰, with some significantly lighter and some heavier than modern seawater’s homogeneous δ60Ni value47. These large variations in δ60Ni values may record variability in Ni sources to the oceans47. It is worth noting that the lightest δ60Ni values reported so far are from the Ni–sulphide ores hosted by Archaean komatiites which have δ60Ni compositions from −0.10 to −1.03‰ (mostly from −0.62 to −1.03‰)38 (Fig. 3). Similar light δ60Ni compositions of −0.82 ± 0.02‰ were reported from the Archaean Ni-rich magmatic sulphides48 (Fig. 3). These exceptionally light δ60Ni values likely reflect Ni isotopic fractionation during magmatic Ni sulphide formation or melting at high temperature38,48.
Environmental significance of the Buchanan Lake data
The δ60Ni values of black shales from the Buchanan Lake section span a very wide range, from −1.09 to 0.35‰ (Fig. 2). The lightest δ60Ni values occur in the oxic interval of the Black Stripe Formation, heavier values toward the anoxic interval of the upper Black Stripe Formation, and slightly heavier in the euxinic interval, suggesting a strong contrast with the isotopic homogeneity of the present-day seawater (Figs. 2 and 3). The δ60Ni values in the lower Black Stripe Formation are among the lightest ever reported for sedimentary rocks; the only reported lighter values are those from magmatic Ni sulphide deposits38,48 (Fig. 3). Of all the known mechanisms potentially responsible for the overall light δ60Ni values from the Buchanan Lake section, the most convincing is pre-EPME input to Sverdrup Basin of Ni sourced from voluminous Ni-rich aerosols released during the STLIP emplacement of nickel sulphide ore deposits. Other explanations for the light δ60Ni values are difficult to support. For example, fractionation during sorption to Mn oxyhydroxide particle surfaces might result in the deposition of isotopically light Ni from seawater. Large Ni isotopic fractionations of up to −3.35‰ caused by surface complexation reactions between the mineral and aqueous phases were reported in laboratory sorption experiments with hexagonal birnessite49. However, the difference between the Late Permian pyritic black shales and Mn oxides indicate that Ni sorption onto MnO2 cannot explain the light δ60Ni values in the black shales. Alternatively, continental weathering of magmatic Ni sulphides could potentially transport relatively light δ60Ni to the oceans compared to the average continental crust. However, there is no occurrence of magmatic Ni–sulphide ores in the Sverdrup Basin and surrounding margins that could be a primary contributor50. Although no Ni isotopic data are available for Late Permian marine hydrothermal fluids, it is unlikely that such fluids would have differed much in δ60Ni throughout the Phanerozoic Eon; there is no reason to expect short-lived changes that would have caused the exceptionally light δ60Ni values in the Buchanan Lake shales. Regardless, there is no evidence of hydrothermal activities in the Sverdrup Basin that could be a source of Ni50.
Several lines of evidence support our interpretation that isotopically light, Ni-rich aerosols came to be a dominant source of Ni input to the Late Permian Panthalassic Ocean. Nickel concentrations in the Black Stripe Formation samples (117.5–247.1 ppm) are well above the 68 ppm Ni for average shale51 (Fig. 2). This Ni enrichment is contemporaneous with episodic coal ash fallout and Hg deposition into the basin, as well as the initial onset of decline in δ13C values (Fig. 2), previously tied to early eruption phases of the Siberian Traps5,11. The age model, based on sedimentation rate, suggests that early onset eruption recorded at Buchanan Lake started ~500 ky prior to the EPME5,11. This timing is remarkably consistent with the initial emplacement of the vertical dike-sill system of the STLIP which is preceded the onset of EPME by at least 300 ± 126 ky (ref. 4). Additional support for a volcanic source comes from the Ni concentration profile recorded in the Noril’sk lava stratigraphy, which shows high Ni concentrations prior to the EPME and a significant drop when the EPME occurred3, as observed in the Buchanan Lake section.
We suggest that the melting and degassing of Ni sulphide during emplacement processes15 may have produced light δ60Ni compositions as a signature of the Siberian Traps, similar to the light δ60Ni compositions measured from magmatic Ni–sulphide (Fig. 3). The Noril’sk ore deposits may be the only known occurrence of a flood basalt-associated magmatic sulphide system that was shallow enough to degas15. Nickel is nonvolatile and normally locked up at depth in magmatic minerals. However, Ni scavenged by magmatic sulphides could have been transferred to magmatic gases, and flotation of Ni-rich sulphide droplets to the surface by gas bubbles may have produced voluminous Ni-rich aerosols15. The global dispersion of Ni-rich aerosols and loading into the Sverdrup Basin, ~20,000 km downwind of the Siberian Traps5, would have been rapid (about 4–8 days) during stratospheric eruptions. The fallout of volcanic Ni emissions would have significantly changed the Ni-isotopic compositions of seawater, resulting in the observed light δ60Ni values in the Black Stripe Formation (Fig. 2).
The δ60Ni increase of 1.23‰ from −0.91 to 0.32‰ during the anoxic interval of the Black Stripe Formation may reflect gradually decreased input of Ni-rich aerosols (Fig. 2). The sharp drop in Ni abundance near the extinction level suggests a greatly reduced loading of Ni-rich aerosols to the Sverdrup Basin during the EPME. It may also reflect the rapid removal of Ni by the expansion of methanogens which resulted in a large pool of methane to the ocean, contributing to the global negative C-isotopic excursions16 (Fig. 2). The sulphidic black shales of the uppermost Black Stripe Formation (−2 to 0 m) have δ60Ni values from 0.07 to 0.34‰, similar to the δ60Ni values of 0.14–0.51‰ for the sulphidic sediments in the modern Black Sea. After the extinction, comparable δ60Ni values of 0–0.34‰ are observed for the sulphidic black shale of the lower Blind Fiord Formation (Fig. 2). The δ60Ni values from the uppermost Black Stripe Formation and lower Blind Fiord Formation suggest that the Ni cycling and isotopic variations were similar to modern Black Sea conditions and consistent with evidence for euxinia at that time5,20,21.
Implications for the end-Permian mass extinction
Though evidence has previously documented remarkable environmental changes near the EPME horizon, our results demonstrate that environmental perturbations triggered by the STLIP magmatism had begun well before the end-Permian mass extinction, and, more importantly, the pathways to environmental exacerbations leading to the EPME are becoming clearer (Fig. 4). Our δ60Ni and Ni content data provide strong evidence for the loading of Ni-rich aerosols into the Sverdrup Basin and a link between eruption of the STLIP, transport of Ni-rich aerosols in the atmosphere, ocean chemistry changes, and mass extinction (Fig. 4). The Buchanan Lake section had a palaeolatitude of 35–40°N from Late Carboniferous to Early Triassic time, and the STLIP eruptions were likely at ~60°N (ref. 19) (Fig. 1C). The superbly preserved Buchanan Lake section in the Sverdrup Basin, Canadian High Arctic, therefore, records unparalleled evidence of environmental changes triggered by the STLIP.
Our data indicate that the voluminous release of Ni-rich gases triggered by the STLIP magmatism had changed the Late Permian ocean chemistry well before the EPME (Fig. 4). Nickel is a bioessential trace metal, and the increase of Ni concentrations in the Sverdrup Basin may have increased primary productivity, which is supported by the steady increase of TOC during the same interval5 (Fig. 4). The increased primary productivity may have depleted oxygen in the water columns, explaining the shift to increasingly anoxic conditions prior to the main extinction (Fig. 4). Further environmental degradation was accelerated by the main eruption of the STLIP near the extinction horizon (Fig. 4). The spike of Hg/TOC ratios near the extinction horizon at Buchanan Lake suggest toxic Hg conditions9,11 (Fig. 4). During the main eruption, the degassing of the sills and volatile-rich sediments of carbonate and coal through contact metamorphism may have liberated extremely high concentrations of iconic greenhouse gases CO2 and CH4, resulting in rapid global warming, ocean euxinia, and catastrophic climate changes4,5,6,7,8,13,14,52,53 (Fig. 4). Sill heating and carbonisation of organic-matter-bearing sedimentary rocks in the Tunguska Basin, as well as the expansion of methanogenic Archaea in the oceans, represent a likely source of 13C-depleted carbon, contributing to the pronounced global negative δ13C excursion at the extinction horizon5,7,8,16,52 (Fig. 4).
Environmental perturbations by voluminous Ni-rich aerosols, coupled with climatic changes driven by greenhouse gases released by the STLIP, such as CO2 and CH4, likely resulted in continuous environmental catastrophes, leading to the end-Permian mass extinction. There is little doubt that Ni isotope measurements on the Buchanan Lake section open a new window on our understanding of the causal link between the STLIP magmatism and EPME, and further Ni isotopic study of the Late Permian—Early Triassic successions worldwide will improve our understanding of global climatic changes and the most devastating biotic crisis in Earth’s history.
Methods
Nickel isotope measurement
Approximately, 100–300 mg of each sample powder was weighted in Savillex screw-top beakers and treated sequentially with distilled 3HF − HNO3, HNO3 + 3HCl, and HNO3. After complete dissolution, solutions were evaporated to dryness at 160 °C and the residues were dissolved in 0.3 N HNO3. The Ni concentration of each solution was determined using an Agilent 7700 quadrupole ICP-MS. Based on the measured Ni concentrations, aliquots of sample solutions containing ~1.5 μg Ni were spiked with a 61Ni–62Ni double spike, in order to reach an optimal spike-sample ratio of 64:36. The spiked solutions were refluxed to ensure sample-spike equilibration before column chemistry.
Separation of Ni from the matrices was achieved using a three-stage, cation exchange chromatography procedure using Bio-Rad 200–400 mesh AG50W-X8 resin32,37. The first column separated Fe, Mn, and Cr from Ni using a mixture of 20% 10 M HCl—80% acetone. The second column used 15% 10 M HCl—85% acetic acid to separate Ni from elements such as Mg, Ca, Al, and Ti. The third column further purified Ni by using 0.9 M HNO3 to remove Na and K. Four USGS standards, Nod-A-1, BIR-1, BHVO-1, and SCO-1 were processed together with samples for column chemistry. The yield was >85% and the total procedural blank was <10 ng, which is negligible compared to ~1.5 μg of Ni in each sample.
Nickel isotopic compositions were measured using a Nu Plasma II MC-ICPMS at Indiana University with an Aridus II desolvating nebuliser. Four Ni isotopes, 58Ni, 60Ni, 61Ni, and 62Ni, were measured simultaneously on Faraday cups. 57Fe was also measured and used to correct for interference on 58Ni, although this correction was always very small. The background for 60Ni was <10−3 V, which is negligible relative to sample signals of ~3–4 V. Each sample solution and the four USGS standards were measured 4 times on different days, and the data processing was done with the Matlab code written by S. Romaniello and described in Wasylenki et al.54. The Ni isotopic data were reported in the conventional δ notation in per mil relative to NIST SRM 986: [δ60/58Ni = (δ60/58Nisample/δ60/58NiSRM986 − 1) × 1000]. The long-term analytical precision based on the analyses on the pure Ni ICP solution is +0.05‰. The four USGS standards yielded δ60/58Ni values of +1.08±0.06‰ for Nod-A-1, +0.17 ± 0.02‰ for BIR-1, +0.05 ± 0.05‰ for BHVO-1, and +0.11 ± 0.04‰ for SCO-1.
Data availability
All data supporting the findings of this study are provided as Supplementary Table 1.
References
Erwin, D. H. Extinction: How Life on Earth Nearly Ended 250 Million Years Ago (Princeton University Press, Princeton, NJ, 2006).
Kamo, S. L. et al. Rapid eruption of Siberian flood-volcanic rocks and evidence for coincidence with the Permian-Triassic boundary and mass extinction at 251 Ma. Earth Planet. Sci. Lett. 214, 75–91 (2003).
Burgess, S. D. & Bowring, S. A. High-precision geochronology confirms voluminous magmatism before, during, and after Earth’s most severe extinction. Sci. Adv. 1, e1500470 (2015).
Burgess, S. D., Muirhead, J. D. & Bowring, S. A. Initial pulse of Siberian Traps sills as the trigger of the end-Permian mass extinction. Nat. Commun. 8, 164 (2017).
Grasby, S. E., Sanei, H. & Beauchamp, B. Catastrophic dispersion of coal fly ash into oceans during the latest Permian extinction. Nat. Geosci. 4, 104–107 (2011).
Fielding, C. R. et al. Age and pattern of the southern high-latitude continental end-Permian extinction constrained by multiproxy analysis. Nat. Commun. 10, 385 (2019).
Svensen, H. et al. Siberian gas venting and the end-Permian environmental crisis. Earth Planet. Sci. Lett. 277, 490–500 (2009).
Svensen, H. et al. Sills and gas generation in the Siberian Traps. Philos. Trans. R. Soc. A 376, 20170080 (2018).
Grasby, S. E., Liu, X., Yin, R., Ernst, R. E. & Chen, Z. Toxic mercury pulses into late Permian terrestrial and marine environments. Geology 48, 830–833 (2020).
Sobolev, S. V. et al. Linking mantle plumes, large igneous provinces and environmental catastrophes. Nature 477, 312–316 (2011).
Sanei, H., Grasby, S. E. & Beauchamp, B. Latest Permian mercury anomalies. Geology 40, 63–66 (2012).
Knoll, A. H., Bambach, R. K., Payne, J. L., Pruss, S. & Fischer, W. W. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313 (2007).
Black, B. A. et al. Systemic swings in end-Permian climate from Siberian Traps carbon and sulfur outgassing. Nat. Geosci. 11, 949–954 (2018).
Shen, Y. et al. Multiple S-isotopic evidence for episodic shoaling of anoxic water during Late Permian mass extinction. Nat. Commun. 2, 210 (2011).
Le Vaillant, M., Barnes, S. J., Mungall, J. E. & Mungall, E. L. Role of degassing of the Noril’sk nickel deposits in the Permian-Triassic mass extinction event. Proc. Natl Acad. Sci. USA 114, 2485–2490 (2017).
Rothman, D. H. et al. Methanogenic burst in the end-Permian carbon cycle. Proc. Natl Acad. Sci. USA 111, 5462–5467 (2014).
Froelich, P. N. et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochim. Cosmochim. Acta 43, 1075–1090 (1979).
Embry, A. F. & Beauchamp, B. Sverdrup Basin. in The Sedimentary Basins of the United States and Canada (ed Miall, A. D.) 559–592 (Elsevier, Amsterdam, 2019).
Beauchamp, B. & Grasby, S. E. Permian lysocline shoaling and ocean acidification along NW Pangea led to carbonate eradication and chert expansion. Palaeogeogr. Palaeoclimatol. Palaeoecol. 350-352, 73–90 (2012).
Grasby, S. E. & Beauchamp, B. Intrabasin variability of the carbon-isotope record across the Permian-Triassic transition, Sverdrup Basin, Arctic Canada. Chem. Geol. 253, 141–150 (2008).
Grasby, S. E. & Beauchamp, B. Latest Permian to Early Triassic basin-to-shelf anoxia in the Sverdrup Basin Arctic Canada. Chem. Geol. 264, 232–246 (2009).
Grasby, S. E., Beauchamp, B., Embry, A. F. & Sanei, H. Recurrent Early Triassic ocean anoxia. Geology 41, 175–178 (2013).
Grasby, S. E., Beauchamp, B. & Knies, J. Early Triassic productivity crises delayed recovery from world’s worst mass extinction. Geology 44, 779–782 (2016).
Grasby, S. E. et al. Isotopic signatures of mercury contamination in latest Permian oceans. Geology 45, 55–58 (2017).
Proemse, B. C., Grasby, S. E., Wieser, M. E., Mayer, B. & Beauchamp, B. Molybdenum isotopic evidence for oxic marine conditions during the latest Permian extinction. Geology 41, 967–970 (2013).
Knies, J., Grasby, S. E., Beauchamp, B. & Schubert, C. Water mass denitrification during the latest Permian extinction in the Sverdrup Basin, Arctic Canada. Geology 41, 167–170 (2013).
Burgess, S. D., Bowring, S. A. & Shen, S.-Z. High-precision timeline for Earth’s most severe extinction. Proc. Natl Acad. Sci. USA 111, 3316–3321 (2014).
Shen, S. Z. et al. Calibrating the end-Permian mass extinction. Science 334, 1367–1372 (2011).
Bruland, K. W. Oceanographic distributions of cadmium, zinc, nickel, and copper in the North Pacific. Earth Planet. Sci. Lett. 47, 176–198 (1980).
Kasting, J. F. Methane and climate during the Precambrian era. Precam. Res. 137, 119–129 (2005).
Konhauser, K. O., Pecoits, E., Lalonde, S. V. & Papineau, D. Oceanic nickel depletion and a methanogen famine before the Great Oxidation Event. Nature 458, 750–753 (2009).
Wang, S. J., Rudnick, R. L., Gaschnig, R. M., Wang, H. & Wasylenki, L. E. Methanogenesis sustained by sulfide weathering during the Great Oxidation Event. Nat. Geosci. 12, 296–300 (2019).
Vance, D. et al. The oceanic budgets of nickel and zinc isotopes: the importance of sulfidic environments as illustrated by the Black Sea. Philos. Trans. R. Soc. A 374, 20150294 (2016).
Wang, S. J. & Wasylenki, L. E. Experimental constraints on reconstruction of Archean seawater Ni isotopic composition from banded iron formations. Geochim. Cosmochim. Acta 206, 137–150 (2017).
Gall, L. et al. Nickel isotopic compositions of ferromanganese crusts and the constancy of deep ocean inputs and continental weathering effects over the Cenozoic. Earth Planet. Sci. Lett. 375, 148–155 (2013).
Cameron, V. & Vance, D. Heavy nickel isotope compositions in rivers and the oceans. Geochim. Cosmochim. Acta 128, 195–211 (2014).
Spivak-Birndorf, L. J., Wang, S. J., Bish, D. L. & Wasylenki, L. E. Nickel isotope fractionation during continental weathering. Chem. Geol. 476, 316–326 (2018).
Gueguen, B., Rouxel, O., Ponzevera, E., Bekker, A. & Fouquet, Y. Nickel isotope variations in terrestrial silicate rocks and geological reference materials measured by MC-ICP-MS. Geostand. Geoanal. Res. 37, 297–317 (2013).
Estrade, N. et al. Weathering and vegetation controls on nickel isotope fractionation in surface ultramafic environments (Albania). Earth Planet. Sci. Lett. 423, 24–35 (2015).
Ratié, G. et al. Nickel isotope fractionation during tropical weathering of ultramafic rocks. Chem. Geol. 402, 68–76 (2015).
Wasylenki, L. E., Howe, H. D., Spivak-Birndorf, L. J. & Bish, D. L. Ni isotope fractionation during sorption to ferrihydrite: implications for Ni in banded iron formations. Chem. Geol. 400, 56–64 (2015).
Ratié, G. et al. Nickel distribution and isotopic fractionation in a Brazilian lateritic regolith: coupling Ni isotopes and Ni K-edge XANES. Geochim. Cosmochim. Acta 230, 137–154 (2018).
Ciscato, E. R., Bontognali, T. R. R. & Vance, D. Nickel and its isotopes in organic-rich sediments: implications for oceanic budgets and a potential record of ancient seawater. Earth Planet. Sci. Lett. 494, 239–250 (2018).
Archer, C., Vance, D., Milne, A. & Lohan, M. C. The oceanic biogeochemistry of nickel and its isotopes: new data from the South Atlantic and the Southern Ocean biogeochemical divide. Earth Planet. Sci. Lett. 535, 116118 (2020).
Gueguen, B. et al. Comparative geochemistry of four ferromanganese crusts from the Pacific Ocean and significance for the use of Ni isotopes as paleoceanographic tracers. Geochim. Cosmochim. Acta 189, 214–235 (2016).
Little, S. H. et al. Towards balancing the oceanic Ni budget. Earth Planet Sci. Lett. 547, 116461 (2020).
Porter, S. J., Selby, D. & Cameron, V. Characterising the nickel isotopic composition of organic-rich marine sediments. Chem. Geol. 387, 12–21 (2014).
Hofmann, A. et al. Comparing orthomagmatic and hydrothermal mineralization models for komatiite-hosted nickel deposits in Zimbabwe using multiple-sulfur, iron, and nickel isotope data. Miner. Depos. 49, 75–100 (2014).
Sorensen, J. V. et al. Large nickel isotope fractionation caused by surface complexation reactions with hexagonal birnessite. Chem. Geol. 537, 119481 (2020).
Davies, G. R. & Nassichuk, W. W. Carboniferous and Permian history of the Sverdrup Basin, Arctic Islands. in Geology of the Innuitian Orogen and Arctic Platform of Canada and Greenland (ed Trettin, H. P.). Geology Survey of Canada, Geology of Canada, (also Geological Society of America) 3, 345–367 (1991).
Wedepohl, K. H. Environmental influences on the chemical composition of shales and clays. Phys. Chem. Earth 8, 305–333 (1971).
Cui, Y. & Kump, L. R. Global warming and the end-Permian extinction event: proxy and modeling perspectives. Earth Sci. Rev. 149, 5–22 (2015).
Elkins-Tanton, L. T. et al. Field evidence for coal combustion links the 252 Ma Siberian Traps with global carbon disruption. Geology 48, 986–991 (2020).
Wasylenki, L. E., Swihart, J. W. & Romaniello, S. J. Cadmium isotope fractionation during adsorption to Mn oxyhydroxide at low and high ionic strength. Geochim. Cosmochim. Acta 140, 212–226 (2014).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (41721002 and 41890842), the 111 project (B14026), Key Research Programme of Frontier Sciences, Chinese Academy of Sciences (QYZDY-SSW-DQC031). L.E.W. was funded by the National Science Foundation (EAR 1929725).
Author information
Authors and Affiliations
Contributions
M.L. and Y.S. conceived the study; S.J.W. and L.E.W. performed Ni isotope analyses; S.E.G. and B.B. collected samples and carried out geological and stratigraphic analyses; M.L. wrote the paper with inputs from S.E.G., S.J.W., X.Z., L.E.W., Y.X., M.S., B.B., D.H., and Y.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Jun Shen, Cécile Quantin and Gildas Ratié for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, M., Grasby, S.E., Wang, SJ. et al. Nickel isotopes link Siberian Traps aerosol particles to the end-Permian mass extinction. Nat Commun 12, 2024 (2021). https://doi.org/10.1038/s41467-021-22066-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-22066-7
This article is cited by
-
Mercury evidence from southern Pangea terrestrial sections for end-Permian global volcanic effects
Nature Communications (2023)
-
Trepostome bryozoans buck the trend and ignore calcite-aragonite seas
Palaeobiodiversity and Palaeoenvironments (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.