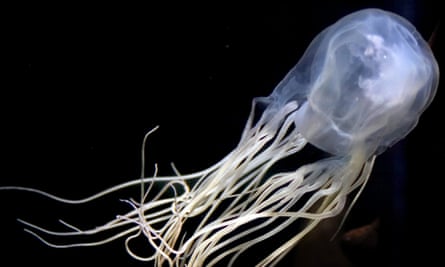
The idea came to Dr Greg Neely after the fruit flies. In May, the Sydney-based scientist and his team of 22 announced they had potentially cured the sting of the box jellyfish, the most venomous creature in the world, whose toxins cause excruciating pain as a best-case scenario, and cardiac arrest as the worst.
It was a simple but groundbreaking technique, using the latest in genetics technology – Crispr, the gene-editing tool that allows scientists to make precise changes to DNA.
The process is fascinating and the implications sprawl across all areas of human health.
Neely’s lab is expanding and looking at a grab bag of human ailments, from cancer to chronic pain. The Canadian-born professor’s field is functional genomics – which is to say, anything to do with genes – with a focus on human health – which is to say, everything. His remit is as narrow as a skin cell but as wide as the concept of pain itself.
He has a floor full of scientists, youngsters with niche and competing interests, international and Australian, sitting in an open-plan office at the University of Sydney’s Charles Perkins Centre.
After de-barbing the jellyfish, they say it is easy to do the same to spiders, snakes and rockfish. They are working on improving the effectiveness of chemotherapy on tumours. They think they have a way to dull chronic pain – in mice at least. Down the line, they hope to work on endometriosis. They are swirling venom up in test tubes in one room, growing spinal cords in another.
Neely’s team can pitch him anything as long as it involves genetics, improves human health, and he finds it “interesting”. This means they can pitch him anything.
“I have been studying pain since 2003,” he says. “The genetics of pain, the molecular mechanisms. How pain happens, and how it is regulated – with the idea of helping people.”

In 2010, Neely used fruit flies to discover 400 new genes, present in humans, that were responsible for feeling pain. The insect has 14,000 individual genes, and until then, he says “the vast majority were not tested for pain”. He found 600. Two-thirds were also in humans. “That was the first step.”
The way Neely’s team created the box jellyfish antidote is simple, once you understand it. They began with human skin cells, floating in test tubes, then added toxic jellyfish venom.
“We have human cells, we add venom and it kills them all,” Neely says. “That’s our starting point. Total death.”
Then with Crispr, they slice and dice the cells, cutting out genes at random. This creates a soup of cells, all with different properties. Some are lacking X, some lacking Y.
“Now we have this big mixture of cells. Then we add our venom, and not everything dies. We keep adding more venom, and not everything dies. Whatever doesn’t die we collect, so it’s a really easy screen.”
It is trial and error; natural selection sped up. “Now [in our new research] we are doing more complex situations,” Neely says. “Not just life and death.
“But this one was great. We picked out all the cells that had resistance, sequenced them and identified which genes were knocked out.”
In the results, published in Nature Communications, the researchers found the resistant cells didn’t have any cholesterol in them. If the skin cells don’t die, then the sting doesn’t hurt – or enter the bloodstream. Their antidote applies a mix of hydrophobic and hydrophilic elements to skin that draws the cholesterol out of the cells. The venom can’t puncture it.
It’s so simple it can go on as a spray. The next step is to find a government to back it, and the required regulatory testing. They are cautious. It has not been tested on humans, and they want to be sure that stopping the pain also stops the other symptoms that affect the heart.
But Neely can’t see any immediate reason why blocking the venom on the skin wouldn’t then block its progress to the heart. He wants it to go through the rigorous testing process.
Since the antidote doesn’t require an injection, or anything similarly invasive, it could be available quickly. “If, after the testing, you told me it was definitely OK,” says Neely, “we could have it for people in a month.”
‘It cures the neuropathic pain’
For Neely, this is just the beginning. The bare process – edit genes, test for resistance, collect – is easily replicable across the whole human body. His team is scurrying into different areas, united by the one goal, broadly, of a pain-free Australia.
The same principle is being applied to cancer. Neely and his team are trying to improve the efficiency of chemotherapy – minimising its harmful impact on good cells, maximising its ability to kill tumours.
They collected 40 of the most commonly used cancer drugs, applied them to a mix of Crispr-edited cells, and saw which ones were resistant and which ones responded.
“Lots of times what happens is that chemotherapy wears off after a while, you get resistance to it,” explains Neely. “So we’re trying to map all the different ways cells can be resistant to chemotherapy.”
Neely speaks with footnotes. He peppers every sentence with references and tributes – to his team, to other researchers. Each sentence starts sprouting tangents, to bring others and their ideas into the story.
They have tested more venoms, he says, waving his hands to different parts of the office. “Raymond has done assassin bug and centipede … We have a plate of 96 venoms that we are testing right now. Sergey is going through and screening some of those. We got them all from Glenn King in Brisbane, who is basically the leader of venom research in Australia.”
He pays tribute to teams at Harvard, a lab in Melbourne run by Marco Herold (“We do the same thing. He’s awesome”), and a Dutch scientist, Thijn Brummelkamp, who he says devised the logic that led to screenings like this.
He waves across the office again. Another member of his team is diving into the puzzle of chronic pain.
“Leslie, she took stem cells and differentiated them into new pain killing neurons, then transplanted them into mice, into the spinal cord. And it basically cures the neuropathic pain in one dose.”
It is a startling discovery. In July, his team published the results in the journal Science Advances. They identified that something similar to chronic pain – a poorly understood condition in humans – potentially affects insects too.
Pain, in a simplistic way, works like a car. When you burn your hand, there are positive messages – that register pain – that travel up your spinal cord. But there are also brakes, negative messages, that soothe it.
“If you slam your hand in your drawer, and you rub it, that is the negative,” Neely says. “And it dulls the pain.”
Fruit flies have the same brake as well, and if the insect suffers a serious injury, Neely’s team discovered this could give them increased sensitivity.
“The sensory nerves get overexcited and they basically over-activate the brakes and it kills the brakes. From now on in their lives, their brakes are gone and they are oversensitive.”
They believe this could be an evolutionary basis for chronic pain, and explain why humans suffer it. “500 million years ago, chronic pain started out as a good thing; it allows you to escape future attacks. And now more recently it sucks for us.”
And early experiments on mice have fixed it – for six months.
“Because these brakes are disappearing, we thought, let’s put them back and see if we can cure pain.
“We take stem cells and turn them into brakes, put them back in the spinal cord and it cures chronic pain.” One injection removes chronic pain for at least six months. They are limited from testing that for longer, due to ethical constraints.
“We now have a grant from the New South Wales government to develop that for humans within three years. That’s a pretty cool new pain therapy.”
The possibilities spool out from this. “One person in my lab just asked if they can start spending time on figuring out the best way to research endometriosis,” Neely says. He told them the first step is to figure out a good, scientifically rigorous gene test.
‘He’s leading the world’
His peers agree that Neely’s work is cutting-edge. The methodology is so clever it generates a kind of excitement.
Prof Mark Hutchinson from the University of Adelaide is a fellow expert in pain, and says the screens Neely is doing are “amazing”.
“Greg is one of Australia’s top pain scientists,” he says. “There is no doubt he is leading the world in this space … it’s amazing. Really cool technology, great insights.”

But, as Neely himself does, Hutchinson notes that there is a difference between pain in mice and fruit flies, and pain in people.
“There is a need to create some translation components to the work. The fruit fly example – and Greg would acknowledge this – the fruit fly is only a model.”
Neely himself shies away from labelling the fruit fly discovery “chronic pain”. In his study, the fly’s pain was measured by how quickly the fly reacted to stepping on a hotplate.
“The definition of pain is an unpleasant emotional and sensory experience – we can’t know if the fruit fly experiences the emotional component,” says Hutchinson. When it comes to chronic pain – itself an already poorly understood, broad, umbrella term – those distinctly human elements are even more important.
“Let’s call it a danger signal rather than true pain,” Hutchinson says. “That is a key element, that Greg used ‘pain’ in inverted commas, which is absolutely the right way to think about it.”
But it’s a groundbreaking first step. “The ability to do a screen like this, from the ethics perspective, being able to use a lower order species like this, it’s a really great advancement,” says Hutchinson. He – like many others in the field – is excited to read Neely’s next paper.
And away from the shadow of the headline-grabbing Crispr, the key breakthrough of Neely’s work is, as Hutchinson says, simply the method, its logical cleanliness. The cleverness of applying such a simple screen to such a complex beast as pain.
Crispr just makes it easier. It’s a tool that lets them isolate those genes with cleaner cuts.
In July, two months after his jellyfish announcement, almost everything Neely was working on has moved forward.
“I just started a collaboration with the UK,” he told the Guardian. “Today, there are 10 or 20 deadly African snake venoms in the mail. We’re basically going to do the same with what we did with jellyfish.”
As always, he has been fielding new pitches from his team.
“When someone pitches a project to me, the first thing I’m doing is thinking why it won’t work. Then if I can’t find any reason why it won’t work, then I next ask, is it important?”
“We are willing to do anything. Taking advantage of new techniques, using the technology we have to help human health. That’s the goal. It’s easier said than done, but that’s the goal.”
